NKG2D immune checkpoint molecule is a part of immune checkpoints of NK cell. The NKG2D receptor, classified as a C-type lectin-like receptor, finds its expression on natural killer (NK) cells, γδ T cells, CD8+ T cells, as well as select autoreactive or immunosuppressive CD4+ T cells. This receptor stands as a pivotal recognition element, playing a central role in identifying and eradicating cells that have undergone damage, transformation, or infection by pathogens.
The NKG2D receptor is composed of a homodimer structure connected by disulfide bonds, which forms an association with a homodimer of DNAX-activating protein 10 (DAP10) to facilitate cellular signaling. NKG2D's ligands encompass MHC class-I polypeptide-related sequence A (MICA), MICB, and a set of six UL16-binding proteins (ULBP1-6). MICA/B molecules, also referred to as PERB11.1/11.2, share analogous structural and functional characteristics. Both molecules feature three extracellular domains (α1, α2, and α3), coupled with a transmembrane domain responsible for cell surface binding.
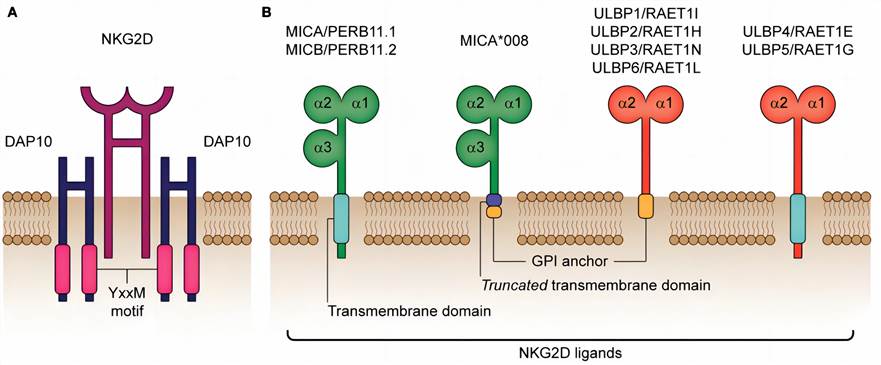 Fig.1. Architecture of the human NKG2D receptor and its corresponding ligands.1,4
Fig.1. Architecture of the human NKG2D receptor and its corresponding ligands.1,4
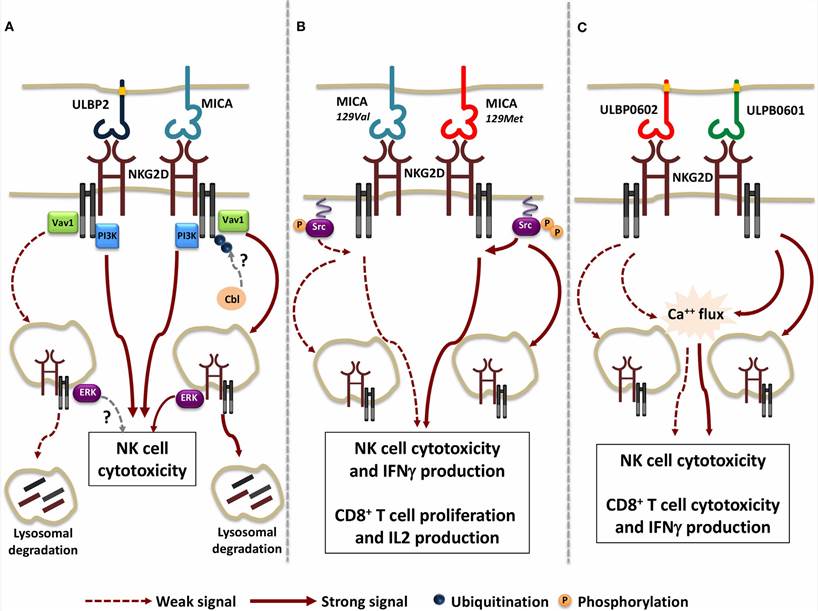 Fig.2. Function resulting from the engagement of NKG2D with distinct ligand/allelic variants.2,4
Fig.2. Function resulting from the engagement of NKG2D with distinct ligand/allelic variants.2,4
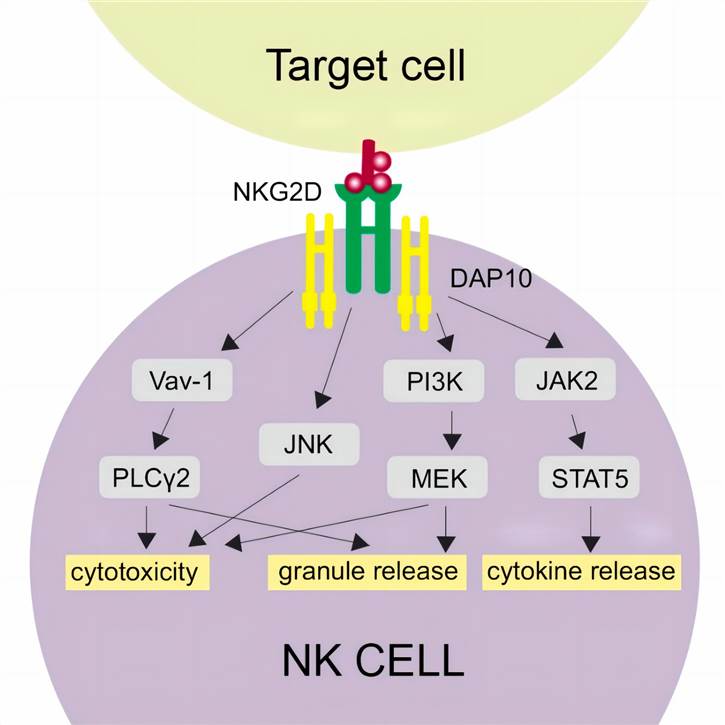 Fig.3. Initiation of NKG2D-associated signaling cascades.3,4
Fig.3. Initiation of NKG2D-associated signaling cascades.3,4
This study groundbreakingly introduces novel immunotherapeutics utilizing camelid-derived single-domain antibodies to effectively target NKG2D, thereby modulating NK cell functions.
These results showcase the operational significance of NKG2D in safeguarding the host against acute viral encephalitis. It achieves this by specifically augmenting cytotoxic T lymphocyte (CTL) efficacy through the infiltration of virus-targeting CD8(+) T cells.
Creative Biolabs offers comprehensive support across a broad spectrum of initiatives within the pharmaceutical frontier, specifically focused on advancing immune regulatory pathways. In the current landscape, we unveil a spectrum of customized resolutions dedicated to the NKG2D immune checkpoint domain, encompassing but not limited to the development of antibodies targeting immune checkpoints, the cultivation of small molecule drugs directed towards immune checkpoints, and the provision of immune checkpoint assays: immune checkpoint assays, immune checkpoint antibody development, biomarker development for immune checkpoint inhibitor (ICI).
We cordially invite our esteemed clients to contact us and share the particulars of your needs.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.