Immune checkpoint drugs present great potential in tumor treatment and have gained a lot of attention in recent years. To meet our clients' demands precisely, Creative Biolabs provides preclinical research for immune checkpoint drug discovery against a variety of tumors. Equipped with novel research tools and extensive experience, we can enable you to free up your time for core work and projects.
The process of drug discovery is time-consuming and costly, which can be typically divided into three major steps: novel drug discovery, preclinical research, and clinical trial. From drug discovery to preclinical research is a continuous process, and the results of preliminary pharmacology and toxicology testing often contribute to lead drug candidate screening. While an Investigational New Drug (IND) application is the boundary between preclinical research and clinical trial. To increase the chances of a successful approval of a new drug, preclinical research is efficient to identify the safety and effectiveness of the drug candidates.
Before testing in humans, the biological effect and safety should be identified via preclinical research, usually include both in vitro and in vivo studies. Pharmacodynamics (PD) always presents the relationship between drug concentration and dose-response, while Pharmacokinetics (PK) presents the absorption, distribution, metabolism, and excretion (ADME) of the drug candidate in the human body. The preclinical toxicology studies provide important information about the suitable and starting dose for further clinical trials. In vitro studies are a simple, fast, and cost-efficient method to understand the mode of action for drug candidates. By contrast, animal model-based in vivo studies can better mimic human disease. The in silico models are a novel method to predict drug candidates' behavior based on computer simulations.
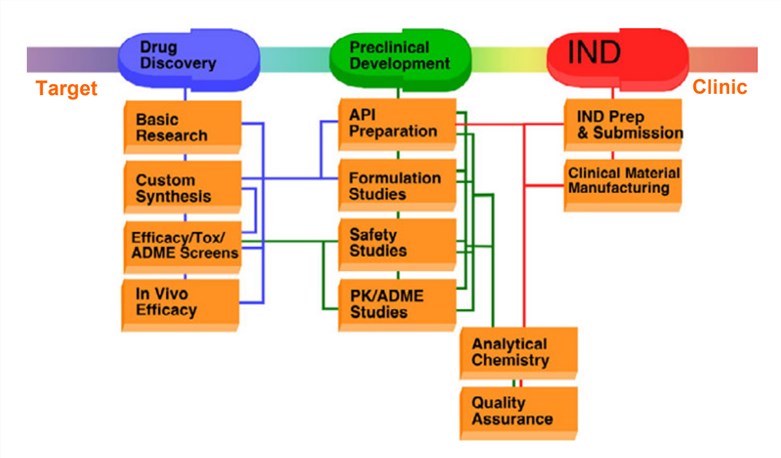 Fig.1 Preclinical drug development stages. (Steinmetz, 2009)
Fig.1 Preclinical drug development stages. (Steinmetz, 2009)
Currently, Creative Biolabs provides optimizing preclinical research approaches to better mimic the complexity of human disease mechanisms for successful drug discovery. Especially, we provide the novel in vivo model development service for immune checkpoint drug discovery against a variety of tumors.
Creative Biolabs has extensive experience in offering novel preclinical services for therapeutic and diagnostic development. We would try our best to provide a customized proposal to meet your project requirements in the quality, timeline, and budget. If you are interested in our services, please do not hesitate to contact us for more details.
Reference
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.