Applications of antibody de novo sequencing in the biopharmaceutical industry include discovering new antibody drug candidates, identifying reagents for research, and determining the primary structure of innovator products for biosimilar development. Based on our advanced technology and abundant experience, Creative Biolabs offers high-quality and customized immune antibody de novo sequencing services to best suit your technical, program, and budget requirements.
The immune checkpoint plays an important role in maintaining immune homeostasis and preventing autoimmunity. During the past decade, immune checkpoint blockade (ICB) therapy has changed the palette of cancer biotherapy. While compared to other proposed immunotherapies such as interferon and cancer vaccines, ICB targeting cytotoxic T lymphocyte-associated molecule-4 (CTLA-4), and programmed cell death receptor-1 (PD-1) / programmed cell death ligand-1 (PD-L1) checkpoints showed more potent and more long-lasting therapeutic effects.
CTLA4 is a member of the immunoglobulin superfamily expressed by activated T cells and transmits an inhibitory signal to T cells. CTLA4 is homologous to the T-cell co-stimulatory protein, CD28, and both molecules bind to CD80 and CD86, also called B7-1 and B7-2, respectively, on antigen-presenting cells. CTLA-4 binds CD80 and CD86 with greater affinity and avidity than CD28, thus enabling it to outcompete CD28 for its ligands. CTLA-4 is well recognized as a key immune checkpoint and has gained significant momentum as a therapeutic target in the field of autoimmunity and cancer.
PD-1 predominantly regulates effector T cell activity within tissues and tumors instead of regulating T cell activation in lymphoid organs. Signals through the PD-1 pathway contribute to the regulation of initial T cell activation, fine-tuning of T cell fate and functions, T cell tolerance, and return to immune homeostasis. Perturbing the PD-1 pathway can profoundly impact host physiology. High and sustained expression of PD-1 and its ligands are common during chronic infections and cancer so that blocking the PD-1 pathway can improve T cell functions and reduce viral load and tumor burden.
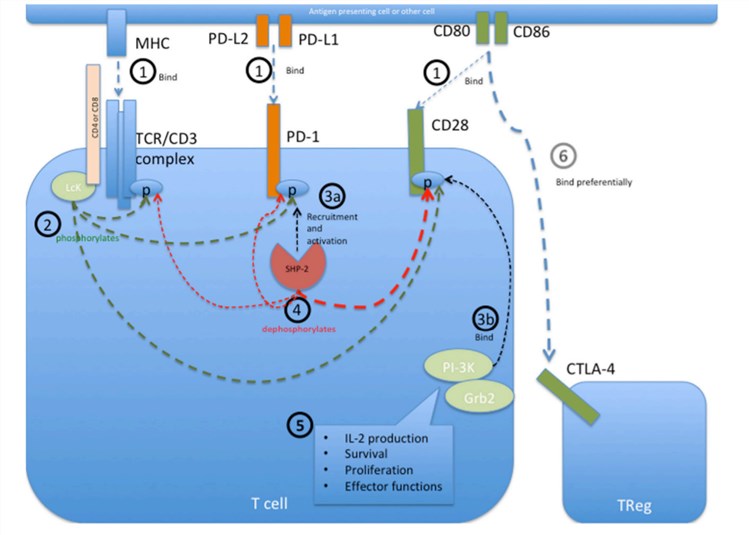 Fig.1. Programmed cell death protein 1 (PD-1) mediated intracellular signaling events during T cell activation.1,2
Fig.1. Programmed cell death protein 1 (PD-1) mediated intracellular signaling events during T cell activation.1,2
Creative Biolabs has an unchallenged ability to achieve 100% accuracy in antibody sequencing based on the proprietary Database Assisted Shotgun Sequencing (DASS) technology. Numerous successful cases from Creative Biolabs have confirmed our qualification to provide antibody sequencing with 100% accuracy and satisfaction guarantee to meet our customers' needs.
As a leading custom service provider in the immune checkpoint field, Creative Biolabs offers the most comprehensive immune antibody de novo sequencing services in response to the requirements of our clients. With multidisciplinary scientists, state-of-the-art instrumentations, and decades of experience in antibody development, we can offer you the highest quality, accuracy, and precision and a fast turnaround to meet deadlines. If you are interested in our services, please feel free to contact us.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.