Stable cell lines have a variety of applications, such as gene functional studies, drug screening, and binding/blocking screening. Immune checkpoints play a significant role in cancer immunotherapy. Based on the common checkpoint proteins, Creative Biolabs has developed high-quality stable cell lines expressing immune checkpoints to accelerate your immunotherapy discovery projects. Our stable cell lines are suited for manufacturing checkpoint antibodies, drug screening, and pharmacological and toxicological studies.
Immune checkpoints are crucial modulators for fine-tuning immune responses. They function as co-stimulatory or inhibitory signals to balance the immune responses. Co-stimulatory immune checkpoints can enhance immune responses, commonly including CD27, CD40, OX40, GITR, 4-1BB, CD28, ICOS, etc. Co-inhibitory immune checkpoints can inhibit immune responses, commonly including PD1, PD-L1, CTLA-4, VISTA, CD155/TIGIT, TIM-3, etc. In recent years, therapeutics that block inhibitory checkpoints or activate stimulatory checkpoints have proved to be powerful agents for restoring anti-tumor immune responses and treating cancers. Immune checkpoint therapy has revolutionized the field of oncology.
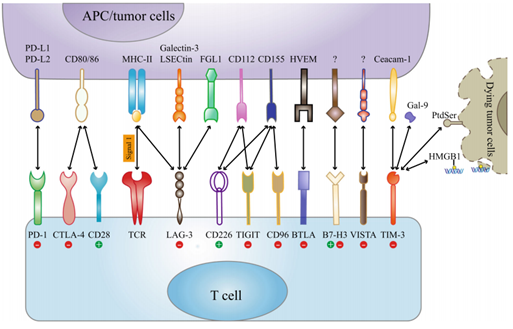 Fig.1. Current and emerging immune checkpoint receptors and their respective ligands.1,2
Fig.1. Current and emerging immune checkpoint receptors and their respective ligands.1,2
As a leader in immune checkpoint therapy development, Creative Biolabs is uniquely positioned to address the demands of stable cell line development with our unparalleled, multiplexed immune checkpoint protein expression platform. Our expert team has years of experience in creating stable cell lines and will work with you to ensure your cell line meets your requirements. Based on the common checkpoint proteins (such as PD1, PD-L1, CTLA-4, VISTA, TIM-3, and LAG-3), we have discovered various stable cell lines to express the related immune checkpoints. Our stable cell lines allow for high-throughput assays (functional assays or binding assays), the overexpression of the respective marker checkpoint protein, and screening high-affinity antibodies against immune checkpoint proteins.

Immune checkpoint stable cell lines can largely facilitate the research of antitumor immunity. Creative Biolabs has developed a series of stable cell line construction technologies and is experienced in cell line development. If you are interested in our services, please feel free to contact us.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.