Creative Biolabs has long-term devoted to the development and application of immune checkpoint inhibitor (ICI) against various tumors. In order to further improve the efficacy and avoid severe immune-related adverse events (irAEs), we now provide novel biomarker development services for immune checkpoint inhibitors (ICIs).
In immuno-oncology, the development of immune checkpoint inhibitors (ICIs) has been a revolutionary milestone to realize more possibilities. As we know, suppressing the anti-tumor immune response by activating immune checkpoints is a major mechanism for tumors to evade immune surveillance. ICIs work by blocking co-inhibitory signaling pathways, allowing the immune-mediated elimination of tumor cells.
For now, there is a variety of ICIs generated for patients with cancers. Nevertheless, the data shows that only a fraction of patients benefit from ICIs, and severe immune-related adverse events (irAEs) are serious limiting factors. According to the inhibition of immune checkpoints, irAEs reinforce the normal physiological barriers against autoimmunity, leading to various local and systemic autoimmune responses. In this case, the development of predictive biomarkers is efficient for better treatment outcomes and fewer irAEs.
-1.png) Fig.1. Schema interaction between tumor and immune cells.1,3
Fig.1. Schema interaction between tumor and immune cells.1,3
Predictive biomarkers can be used to determine the effectiveness of the therapy before initiating the treatment. In addition, these biomarkers play an important role in the design of treatments, such as indicating whether the patient will benefit from a specific checkpoint monotherapy or whether combination therapy is required.
In general, the biomarkers could be classified into two groups: tumor-derived immune biomarkers and immune cell-derived biomarkers. Several tumor-derived biomarkers have been reported, including PD-L1 expression on tumor cells, neoantigens, and high tumor mutational load. The immune cell-derived biomarkers present great correlation to the clinical outcomes, such as increased PD-L1 expression on immune cells, the presence of TILs in tumor microenvironments, and the ratio of effector CD8+ T cells to FoxP3+ regulatory T cells in tumors.
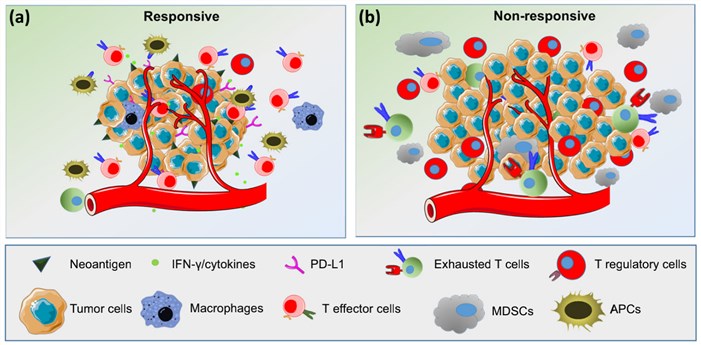 Fig.2. Overview of predictive biomarkers for response to ICIs.2,3
Fig.2. Overview of predictive biomarkers for response to ICIs.2,3
Creative Biolabs is a leading service provider with many years of experience in ICIs biomarker development. We would be pleased to provide support for any of your questions. If you are interested in our services, please do not hesitate to contact us for more details.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.