Immune checkpoints consist of inhibitory and stimulatory pathways that maintain self-tolerance and assist with immune response. In cancer cells, immune checkpoint pathways are often activated to inhibit the nascent anti-tumor immune response. Immune checkpoint-blocking antibodies have come to the forefront of cancer immunotherapies as a powerful and promising strategy to activate the immune system.
With our state-of-the-art technology and industry-leading expertise, Creative Biolabs has accumulated extensive experience in monoclonal antibody (mAb) and recombinant antibody discovery and development. Our professional technical scientists, comprehensive, robust production systems, and abundant experience make us a perfect partner to help our clients with immune checkpoint antibody production. We are committed to providing overall solutions for immune checkpoint antibody production and manufacture by delivering custom services to meet our clients' R&D timeline and budget.
Creative Biolabs is well equipped and versed in immune checkpoint antibody production and manufacture. Our antibody production services include both production and purification of multi-isotypes antibodies, including antibodies with full-lengths, such as human IgGs (IgG1, IgG2, IgG3, IgG4), mouse IgGs (IgG1, IgG2a, IgG2b, IgG3), rat IgGs (IgG1, IgG2a, IgG2b, IgG2c), rabbit IgG, canine IgG, IgA, IgM and IgE, and antibodies with partial lengths, such as scFv, Fab, F(ab)2 and bsAb. By leveraging the wealth of information that we have on immune checkpoint antibodies, we provide the most suitable services to meet our clients' needs. Our years of experience and flexible service options for small-scale production and large-scale production can help deliver fast, reliable support for any phase of our clients' immune checkpoint antibody production projects.
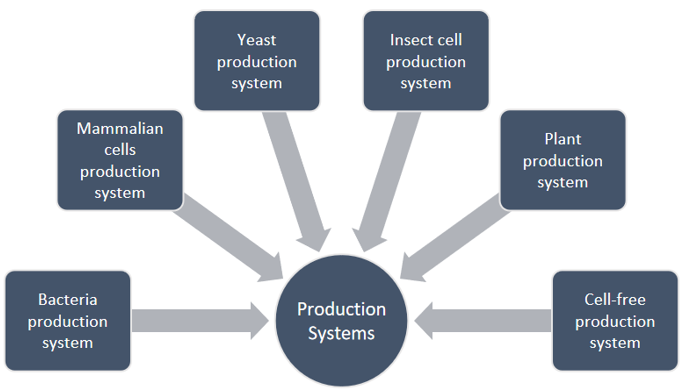
Creative Biolabs has established several Escherichia coli systems for antibody production for secreting expression in the periplasm, soluble expression in the cytoplasm, and insoluble expression as inclusion bodies, such as high expression systems with T7 RNA polymerase gene promoter, coexpression of foldases, or molecular chaperones to prevent misfolding and aggregation of antibody fragments.
Creative Biolabs offers mammalian cell transient expression and stable cell line development for the production of immune checkpoint antibodies with a volume that could reach 100 L and even more.
Creative Biolabs has engineered yeast cells (Saccharomyces cerevisiae and Pichia pastoris) for enhanced production of immune checkpoint antibodies.
Due to the unique glycosylation patterns of insect cells, Spodoptera frugiperda (SF) and Trichoplusia ni are the primary insect cell lines used for antibody production. Insect cells are available at Creative Biolabs to support the production of immune checkpoint antibodies.
Due to the lack of animal pathogenic contaminants, low production cost, and ease of agricultural scale-up of plant expression systems, plants are considered a potential alternative. Creative Biolabs has established an advanced Nicotiana benthamiana- and N. tabacum-based antibody production platform to produce immune checkpoint antibodies.
Based on the cell lysate of prokaryotic or eukaryotic cells, Creative Biolabs provides several cell-free systems for the production of immune checkpoint antibodies.
Creative Biolabs is fully competent and dedicated to serving as your one-stop-shop for immune checkpoint antibody development. If you are interested in immune checkpoint antibody production, please do not hesitate to contact us for more detailed information.
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.