Tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2), an intracellular adapter protein, was initially identified as a protein that interacts with TNF receptor 2 (TNFR2), but it also interacts with a wide range of other signaling proteins, including plasma membrane receptors, kinases, phosphatases, other E3 ligases, and deubiquitinases.
TNFR2 has four cysteine-rich domains (CRDs). As a pre-ligand binding assembly domain, the first CRD of TNFR2 (CRD1) facilitates low-affinity (relative to ligand affinity) receptor molecule autoassociation. CRD2 and CRD3 participate in ligand binding. CRD4 has not yet been related to a specific function.
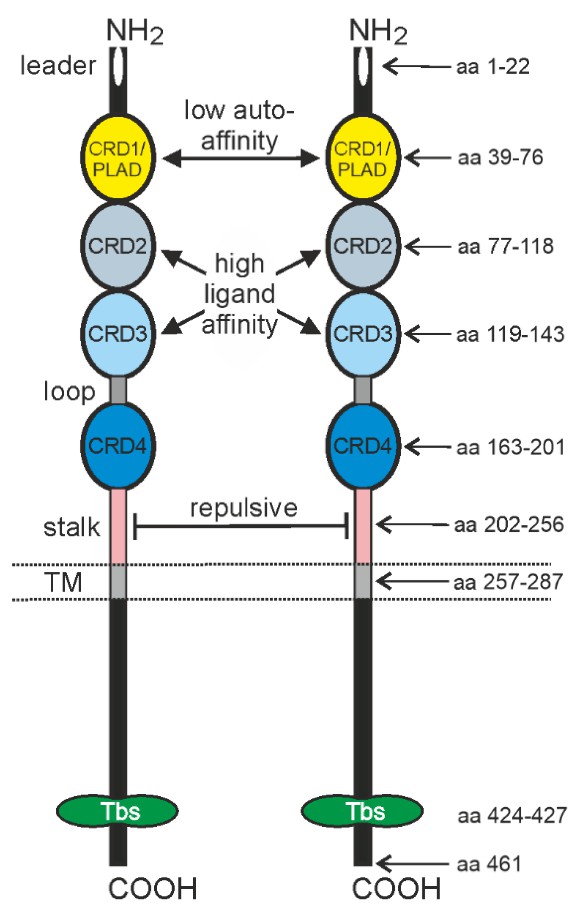 Fig.1. Domain architecture of TNFR2.1,3
Fig.1. Domain architecture of TNFR2.1,3
TNFR2 is expressed on a wide range of malignancies and malignant cells and accumulates greatly in the tumor microenvironment. Increased TNFR2 levels will promote the growth of tumor cells. Regulatory T-cells (Tregs), regulatory B-cells (Bregs), myeloid-derived suppressor cells (MDSCs), and cytotoxic CD8+ T effector cells (Teffs) are among the immune cell types that also express TNFR2.
TNF recruits TNF receptor-associated factor 2 (TRAF2), TRAF2-associated proteins, and apoptosis-related enzymes to activate TNFR2. TNFR2 subsequently stimulates NF-B, STAT5, YAP, and other transcription factors through several pathways to promote transcription of its target genes, preventing tumor cell death and stimulating tumor cell growth. TNFR2 also participates in numerous alterations in the tumor microenvironment via signal transduction such as JUNK, MLCK, and EGFR2. TNFR2 promotes tumor immune escape by virtue of its ability to stimulate various immune suppressive cell types and can act as an oncogene.
Besides working as a T cell stimulator, increasing the activity and phenotypic stability of tumor-infiltrating regulatory T cells (Tregs), TNFR2 may also have a suppressive or stimulatory effect in the tumor microenvironment by regulating numerous immune cells.
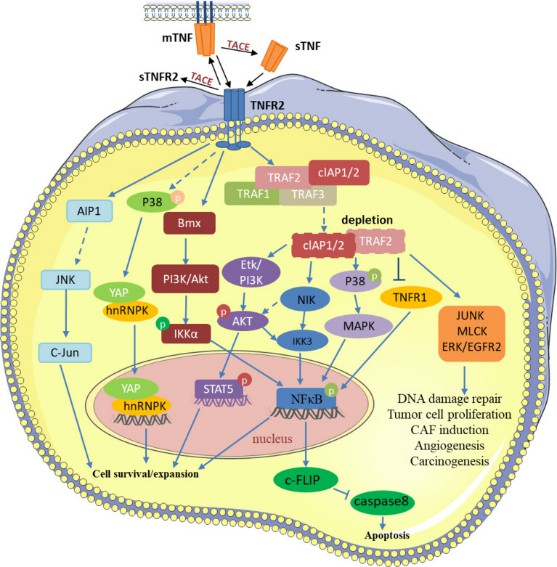 Fig.2. TNFR2 related signaling pathways in the tumor.2,3
Fig.2. TNFR2 related signaling pathways in the tumor.2,3
As a novel and prospective target, TNFR2 agonism and antagonism play critical roles in autoimmune and tumor microenvironments. According to this characteristic, several anti-TNFR2 agonist antibodies have been discovered to increase the activity of effector T cells and some antagonist antibodies that prevent TNF from binding to TNFR2 and prevent the conversion of mTNFR2 to sTNFR2.
There are also some agonistic antibodies against TNFR2. Recently, a new type of anti-TNFR2 antibody was reported. They can recognize the receptor outside the TNF-binding domain, expanding the population and boosting the functionality of CD8+ T cells while not affecting the suppressive function of Tregs and changing the ratio of CD8+ T cells to Tregs in vitro when mediated by CD8+ T cells and NK cells. Moreover, the combination of anti-TNFR2 antibody and other immune cell agonists can synergistically suppress tumors.
TNFR2 has tremendous potential in tumor immunotherapy, and various antibody-based TNFR2 agonists and TNF antagonists have been proposed with strong clinical practice potential. Creative Biolabs provides a comprehensive range of drug development services that target the immunological checkpoint TNFR2.
Please contact us for further information if you are interested in any of our services.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.