Adenosine is a critical immunosuppressive metabolite in the hypoxic microenvironment of tumor tissue and is generated from ATP in intracellular and extracellular compartments. It involves the regulation of various physiological processes and is essential in protecting an overzealous immune response during inflammation and tissue damage. Adenosine plays an anti-inflammatory role by binding to four different types of G-protein-coupled adenosine receptors (A1R, A2aR, A2bR, and A3R).
The high-affinity A2A adenosine receptor (A2aR) is one of the most important factors in this pathway and is mainly activated by adenosine. A2aR is highly expressed on various immune cell subsets and endothelial cells, including T cells, APCs, NK cells, and endothelial cells. A2aR has been shown to protecting tissues during inflammatory responses. In the context of cancer, the accumulation of extracellular adenosine binding to its ligands A2aR suppresses antitumor immune responses.
While several immune checkpoint pathways are triggered by membrane-bound ligands (such as LAG-3 and TIM-3 pathways), there are also some soluble ligands found in the immune microenvironment that can function as triggers for checkpoint pathways, especially extracellular adenosine and its receptor A2aR, which are identified as critical elements in immune regulation. A2aR-mediated adenosine signaling is an intrinsic negative regulator of NK cell maturation and anti-tumor immune responses. In the tumor microenvironment (TME), A2aR signaling not only prevents an effective anti-tumor immune response by inhibiting the infiltration and function of immune effector cells such as CD8+ T cells, natural killer (NK) cells, and DC cells but also enhances the proliferation and polarization of immunosuppressive T regulatory cells, thereby promoting the progression of neoplasm. Therefore, blocking A2aR activation has shown the potential to enhance anti-tumor immunity markedly in preclinical studies.
To learn more about the A2aR-involved pathway, please visit A2aR and adenosine pathway.
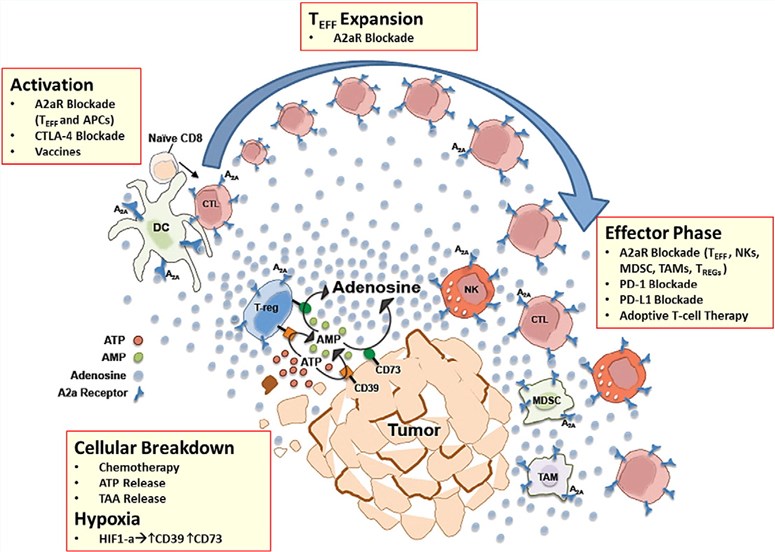 Fig.1. A2aR blockade.1,2
Fig.1. A2aR blockade.1,2
Given the similarities between A2aR-mediated immune modulation and established checkpoint pathways (such as CTLA-4 and PD-1), the application of A2aR blockade to tumor immunotherapy is particularly exciting in recent years. Blockade of adenosine generation and signaling via the A2aR (predominantly expressed on immune cells) increases the therapeutic efficacy of clinically approved immunotherapies in preclinical models. As a case in point, the recent study found that A2aR blockade has distinct effects on T cell activation in a mouse melanoma model. Additionally, A2aR blockade will also promote tumor immunotherapy through its effect on NK cells and tumor-associated macrophages.
To date, several studies strongly support the development of A2aR antagonists as novel modalities in the immunotherapy armamentarium. Moreover, A2aR blockade has been combined with other immunotherapy approaches to potentiate additive effects on tumor control in a number of studies. Recently, some A2aR antagonists are undergoing clinical trials either alone or in combination with other immunotherapies.
As a leading CRO for immune checkpoint drug development, Creative Biolabs is the premier provider to offer state-of-the-art supports for our customers worldwide. Currently, a series of custom services for immune checkpoint A2aR is available at Creative Biolabs, including but not limited to:
If you are interested in more detailed information, please do not hesitate to contact us.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.