Fibrinogen-like protein 1(FGL1), also known as LFIRE-1, HFREP-1, or HPS, is a type of protein belonging to the fibrinogen family, that is comprised of a disulfide bond-linked homodimer and released by the liver and pancreas. FGL1 is not only involved in hepatocyte mitosis and hepatic energy utilization (including lipid metabolism and blood glucose regulation) but also plays a role in other tissues such as muscle and brown adipose tissue. For instance, FGL1 regulates body production and temperature by acting on brown adipose tissue via blood circulation, FGL1 also affects muscle tissue, influencing myoblast insulin sensitivity.
FGL1 expression is shown to be increased in lung, prostate, melanoma, colorectal, breast cancer, and brain tumors, but decreased in pancreatic, breast, liver, and head and neck malignancies. Tumor epithelial to mesenchymal transition (EMT) is linked to tumor cell invasion and migration, and multiple studies show that FGL1 mediates the EMT process in tumors. Besides, FGL1 is involved in the regulation of cell proliferation via the AKT-mTOR signaling pathway, as well as the regulation of apoptosis via the PARP1/caspase-3 pathway. Tumor cell sensitivity to EGFR-targeted tyrosine kinase inhibitors is also linked to FGL1.
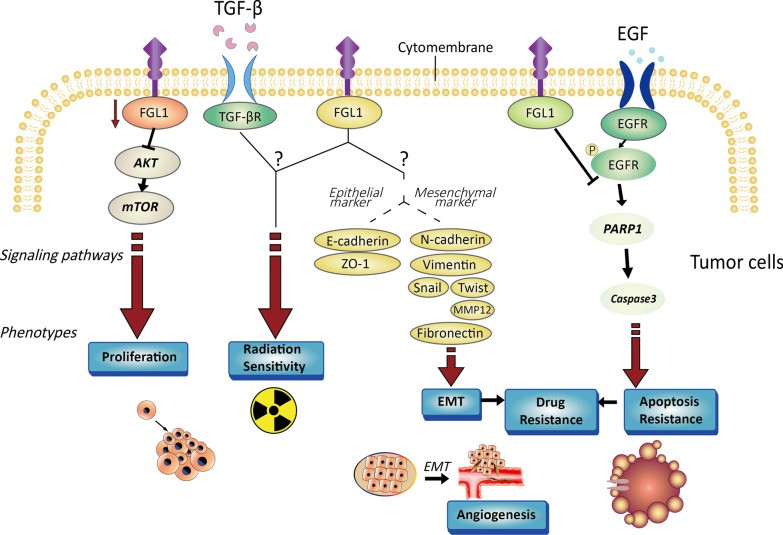 Fig.1. Characterization of tumor cells controlled by FGL1.1,2
Fig.1. Characterization of tumor cells controlled by FGL1.1,2
FGL1 is a high-affinity immunosuppressive ligand that binds to LAG3. Loss of the FGL1/LAG3 connection might be induced through gene deletion or antibody blockade, and antitumor immunity could be increased by boosting tumor-infiltrating lymphocyte (TIL) activation and growth in the TME. Inhibiting FGL1 has been shown to work in tandem with anti-PD-1/PD-L1 therapy to suppress the effect of PD-1/PD-L1 signaling. Moreover, the FGL1/LAG3 pathway also performs an immunosuppressive effect independent of the PD-1/PD-L1 pathway, both FGL1/LAG3 and PD-1/PD-L1 can regulate T cells separately, and inhibiting both of these checkpoints can cause synergistic antitumor effects.
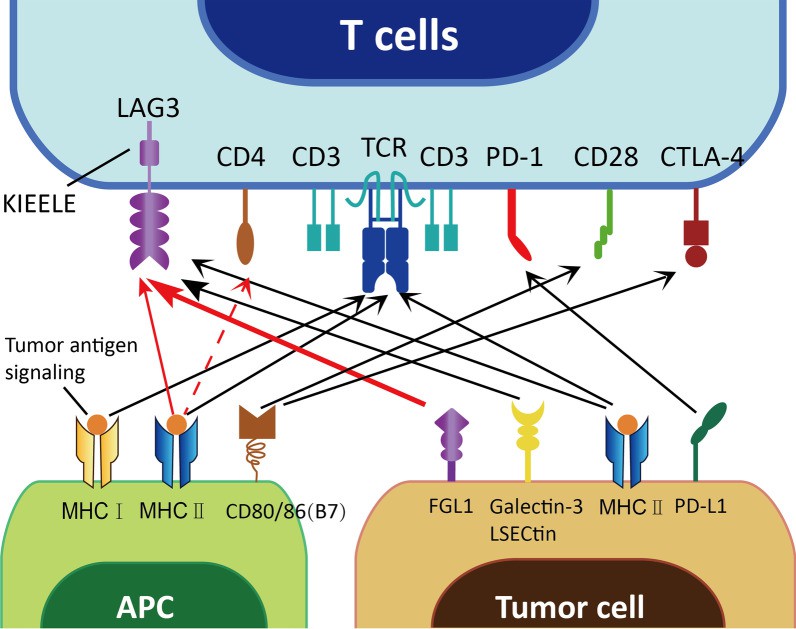 Figure 2 FGL1 binds to LAG3.1
Figure 2 FGL1 binds to LAG3.1
Based on the FGL1/LAG3 pathway, anti-FGL1 can be a promising new checkpoint for overcoming cancer immunotherapeutic resistance. Study reveals that using FGL1 drugs can boost T cell antitumor activity and thereby impact ICI-acquired resistance in patients with varying degrees of PD-L1 expression. Antigen presentation inhibition is one of the reasons for ICI resistance. FGL1 has the capacity to produce ICI resistance in a receptor-ligand-dependent way. The level of FGL1 secretion in plasma can be used to identify patients who will not benefit from ICI treatment. In addition, a substantial favorable link between FGL1 expression and long-term prognosis has been seen in patients with multiple forms of metastatic cancer who are treated with anti-PD-1/PD-L1 treatment.
FGL1 is thought to be a novel immune checkpoint molecule with a bright future in clinical applications. Creative Biolabs provides immune checkpoint FGL1-related services to help your immunotherapy research progress.
If you have any further questions, please contact us.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.