In recent decades, chemotherapy is the only approach for patients with metastatic tumors, which always leads to severe adverse events as well as high rates of relapse. The immune cell activation has been served as the most effective approach for the activation of anti-tumor immune responses and cancer treatment. The studies have shown that combined immune checkpoint blockade (ICB) provides unprecedented efficacy gains in numerous cancer indications. Compared with either monotherapy, the combined immune checkpoint therapy is sufficient to induce unique cellular responses for better efficacy.
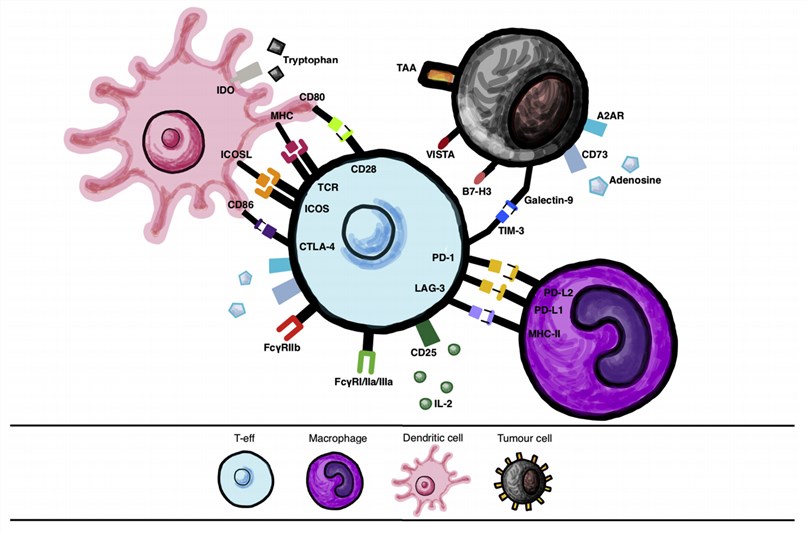 Fig.1. Immune cell interactions via checkpoint molecules and their ligands.1,2
Fig.1. Immune cell interactions via checkpoint molecules and their ligands.1,2
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) are classical inhibitory checkpoints for cancer treatment. The combination of CTLA-4 and PD-1 blockers presents increased response rates and survival rates in multiple cancer types.
lymphocyte activation gene-3 (LAG-3) has been served as the foremost target next to PD-1 in the development of cancer therapy. As an inhibitory coreceptor, LAG-3 plays an important role in autoimmunity, tumor immunity, and anti-infection immunity. LAG-3 expression is correlated with CTLA-4 expression on tumor-infiltrating lymphocytes (TILs).
Indoleamine 2'3' dioxygenase (IDO, IDO1, and IDO2) is a catabolic enzyme that promotes immune tolerance. The combination treatment with anti-CTLA-4 and IDO inhibitors avoids the resistance and tumor growth derived from a single anti-CTLA-4 or anti-PD-1 treatment.
The combination immunotherapy targeting PD-1 and LAG-3 would encourage tumor-specific response and avoid the self-antigen-specific or non-specific immune response. The preclinical data showed that LAG-3 is synergistically efficacious in combination with anti-PD-1 therapies.
TIM-3 is the exhaustion marker in cancer. Compared with a single PD-1 blockade in cancer models, the studies have shown that the combined TIM-3/PD-1 blockade presents superior tumor regression.
TIGHT is the novel checkpoint inhibitor in cancer immunity. Some studies have shown that the anti-PD-1 and anti-TIGIT combination therapy enhances antitumor immunity and survival in murine glioblastoma (GBM) models.
The combined therapy targeting both PD-1 and CD47 activates both innate and adaptive immune responses against multiple tumors. This approach can not only maximize the anti-tumor therapeutic effect but also elicit more durable responses.
Compared with the treatment with PD-L1 and 4-1BB mAb alone, the dual treatment induced further tumor regression and enhanced survival. The number of tumor-infiltrated CD103+ CD8+ T cells is significantly increased for improved antitumor efficacy.
The combination immunotherapy targeting PD-1 and GITR decreases CD8+ T cell dysfunction and induces a highly proliferative precursor effector memory T cell phenotype with a CD266-dependent manner.
V-domain Ig suppressor of T cell activation (VISTA) is the co-inhibitory receptor homolog with PD-L1. VISTA functions for T cell activation through nonredundant functions distinct from the PD-1/PD-L1 pathway.
The adenosine-adenosine A2A receptor (A2AR) pathway is one of the important targets for immunotherapy. There are a series of A2AR/CD73 inhibitor clinical trials for multiple cancer treatments.
The combination of CTLA-4 blockade with 4-1BB activation improves tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production.
The expression of B7-H3 in melanoma results in favoring tumor growth and conferring anti-apoptotic processes. The combination of CTLA-4 mAb with B7-H3 mAb has been the subject of a phase I trial.
In liver cancer, the combination treatment of GITR-ligation and CTLA-4 blockade enables the alleviation of immunosuppression mediated by Ti-Treg. Compared with either treatment alone, the combined treatment presents a stronger recovery of T cell function.
Studies have shown that combined anti-OX40/anti-CTLA-4 immunotherapy increased the effector CD4 and CD8 T cell expansion and differentiation, which results in enhanced survival of mice with prostate or sarcoma tumors.
Anti-CD73 mAb significantly enhanced the activity of anti-PD-1 mAb against colon and prostate subcutaneous tumors. The combined blockade of CD73 ad PD-1 can enhance the therapeutic activity for improved therapeutic strategies.
In the mice bearing human PD-L1-expressing colon carcinoma MC38 tumors, the anti-PD-L1 antibody can significantly limit tumor growth, which is further enhanced by the combination inhibition with anti-TGF-β, leading to a 66.7% tumor regression.
Intravenously administered single or combination of anti-B7-H3 or anti-PD-1 antibodies are given ten days following the breast cancer cell implant. The treatment with anti-B7-H3 or anti-PD-1 alone has no discernible anti-tumor effects from the results of the living bioluminescence image.
Individual tumor growth curves in the breast cancer mouse model reveal that tumors are only moderately rejected by anti-PD-L1 or anti-4-1BB mAb, with pronounced tumor delay in the combination therapy.
The ability of transplanted cells to normally express CD47 and PD-L1 in vivo is demonstrated by immunofluorescent staining. These mice are then given monoclonal antibodies of anti-CD47 and anti-PD-L1 separately or in combination to assess their anti-tumor effectiveness.
The tumors in the mouse model subcutaneously transplanted with tumor cells show a reduction in tumor size and an increase in survival rate when delivering anti-PVRIG. This anti-tumor activity is functional in a lymphocyte-dependent manner and weakens when NK cells and CD8+ cells are depleted, reflected by the increasing tumor volume.
CD96 can block the binding of PVR in an identical manner with TIGIT. Flow analysis illustrates the expression of PVRIG, TIGIT, and CD96 on CD8+ T cells and PVRL2 and PVR on the Mel-624 malignant melanoma cell line, respectively.
Another research group illustrates the underlying mechanism of combined immune checkpoint treatment between KLRG1 and PD-1. Similar results are generated in the melanoma mouse model, regarding the synergistic effect on reduced tumor volume.
B7-H3 is also highly expressed in the pancreatic ductal adenocarcinoma cell line Pan02, which provides the opportunity for combined immune checkpoint therapy based on anti-B7-H3 and anti-PD-L1.
Creative Biolabs is a leading service provider that focuses on immune checkpoint therapy especially combined immune checkpoint therapy against multiple cancers. Based on our advanced drug discovery platform and extensive experience, now we can provide a series of services for our clients all over the world, which include but not limited to:
If you are interested in our services, please do not hesitate to contact us for more detailed information.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.