In recent years, there has been notable advancement in the field of cancer treatment with the use of immune checkpoint inhibitors (ICIs). Nevertheless, a substantial portion of patients fail to achieve sustained and optimal clinical outcomes through immunotherapy alone. Creative Biolabs regards the exploration of combination regimens as a novel and dynamic research focal point.
MicroRNAs (miRNAs), a class of endogenous small non-coding RNAs consisting of 18 to 25 nucleotides, play pivotal roles in the regulation of both physiological and pathological processes. Mounting evidence supports their function as pivotal immune modulators in the context of tumors. Notably, miRNAs, whether acting as tumor suppressors or oncogenes, have been observed to modulate anti-tumor immunity or facilitate communication between tumor cells and the immune milieu.
Furthermore, it is worth highlighting that a single miRNA can target multiple checkpoint molecules, mirroring the therapeutic effect of combined immune checkpoint blockade (ICB). Consequently, miRNA therapy is being considered as a strategy to enhance the efficacy of ICIs. Moreover, miRNAs hold promise as predictive and prognostic biomarkers, as well as therapeutic targets in the realm of immunotherapy.
A single miRNA has demonstrated its ability to simultaneously target numerous checkpoint molecules, mirroring the therapeutic impact of a combined approach to ICB. The integration of microRNA therapy with ICB holds the potential to enhance the effectiveness of the established monotherapeutic paradigm.
|
|
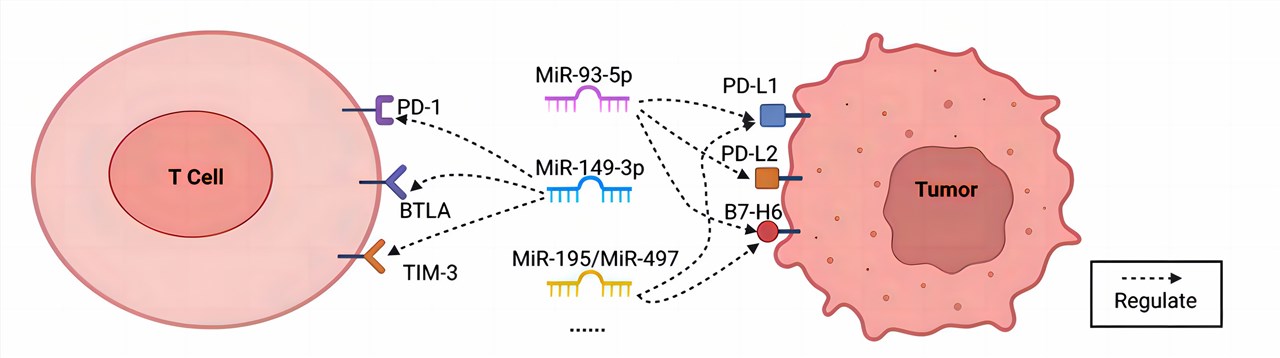
| Fig.1. Key microRNAs orchestrating multifaceted immune checkpoint control.1,2 | |
| Contrary to earlier knowledge of miRNA primarily governing tumor cell biology, current research has unveiled a novel perspective wherein tumor-associated miRNAs exert influence over anti-tumor immune responses. Furthermore, these miRNAs have been found to mediate intricate interplays between tumor cells and the surrounding immune milieu, shedding light on the mechanisms responsible for their dysregulation during the course of tumorigenesis. | |
| MicroRNAs orchestrate diverse facets of the anti-tumor immune response, encompassing immune checkpoint molecules (PD-1, PD-L1, and CTL-A4), immune cell populations (macrophages, MDSCs, and NK cells), and the machinery governing tumor antigen processing. These findings suggest that miRNAs may represent the pivotal connecting element in the quest for potential biomarkers capable of prognosticating the efficacy of immune checkpoint inhibitors in tumor responses. | |
Creative Biolabs provides a comprehensive range of tailored services pertaining to immune checkpoints, encompassing, yet not confined to: Immune Checkpoint Antibody Development, Immune Checkpoint Assays, Immune Checkpoint Targeted Peptide Development, etc. Please contact us for a thorough understanding.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.