In the pursuit of harnessing the full potential of immune checkpoint therapy, Creative Biolabs is devoted to a relentless endeavor aimed at surmounting the formidable challenge of drug resistance. This formidable task necessitates the exploration of promising therapeutic avenues. Guided by insights into anti-tumor immune responses, strategies devised to conquer resistance to immune checkpoint therapy can be classified into the following facets: fortifying antigen processing and presentation, bolstering the vigor and infiltration of T cells, and dismantling the stifling immunosuppressive microenvironment. Currently, numerous combination therapies are undergoing rigorous assessment in both preclinical models and clinical trials.
Immune checkpoint therapy has gained increasing prominence in clinical applications, demonstrating therapeutic efficacy across various human cancers. Nonetheless, drug resistance to ICIs remains a prominent impediment for immunotherapy patients. Creative Biolabs is actively engaged in numerous studies aimed at unraveling the functional mechanisms underpinning resistance to ICIs, research has illuminated the propensity for resistance to manifest at various stages within the tumor's immune response continuum, as delineated below:
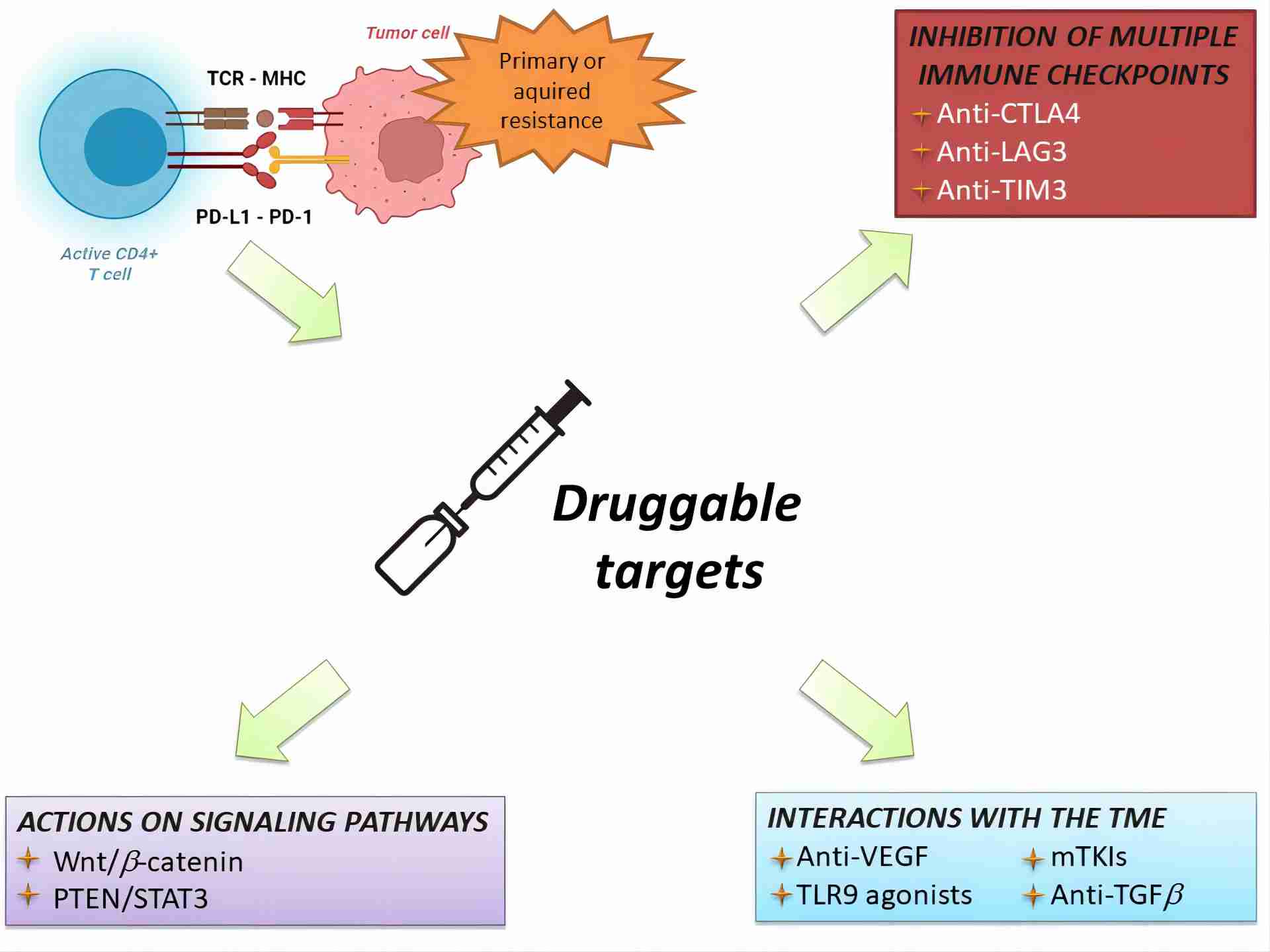
|
Fig.1. Current therapeutic approaches for overcoming immunotherapy resistance.1,2 Hepatocellular carcinoma (HCC), the predominant form of liver cancer globally, poses a significant health challenge. While ICIs stand as the foremost systemic therapy for HCC, around 40% of patients face primary resistance, failing to achieve disease control. Furthermore, a similar proportion experiences disease progression following an initial response due to secondary resistance. This review outlines the mechanisms underlying both primary and secondary resistance and provides insights into the ongoing therapeutic approaches aimed at surmounting these challenges. |
| The utilization of ICIs has yielded remarkable long-term survival outcomes in non-small cell lung cancer (NSCLC). Nevertheless, a majority of patients eventually develop resistance to ICI therapy. The emergence of ICI resistance is orchestrated by a myriad of intricate mechanisms, impacting various aspects, including but not limited to, tumor cell-intrinsic modifications and the tumor microenvironment. The potential to modulate the immune response through targeted intervention with alternative immune receptors, pathways, and mediators offers a promising avenue to prolong or forestall the onset of resistance. | |
Creative Biolabs is a renowned authority that provides comprehensive support for an array of immune checkpoint-related drug development initiatives. Presently, we offer an extensive suite of customized services dedicated to tackling resistance in immune checkpoint therapy. These services encompass, but are not confined to: Immune Checkpoint Targeted Peptide Development, Immune Checkpoint Targeted Small Molecule Drug Development, and Biomarker Development for ICI. Our proficient team is poised to optimize the process and enhance efficiency by providing personalized customer service tailored to your specific needs. For further information, please feel free to contact us.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.