The progression of cancer is regulated by the surrounding tumor microenvironment (TME), which is commonly metabolically stressed and immunosuppressive, comprising tumor cells, fibroblast cells, vascular endothelial cells, and various types of innate and adaptive immune cells, together with extracellular matrix (ECM) as well as multiple extracellular soluble molecules (including cytokines, growth factors, chemotactic factor, etc.). There are multiple key factors within TME that modulate escape from the surveillance of the host immune system, contributing to a dynamic environment with inherent complexity during cancer development or on different therapeutic regulations.
The TME could be characterized according to tumor T cell infiltration, into "cold" or "hot", which is also involved with the levels of pro-inflammatory and cytokine production. "Cold tumors" with lack of tumor T cell infiltration are also a major factor related to initial resistance to immune checkpoint therapy, while "hot tumors" keep a better response rate to ICB treatment. Therefore, converting a "cold" microenvironment to a "hot" one will increase the sensitivity of tumors to concurrent or subsequent immunotherapy treatment, and can be considered an auspicious approach to overcoming resistance to ICBs.
According to the steps of immune response, the paucity of tumor T cell infiltration can be attributed to the lack of tumor antigens, APC deficit, absence of T cell priming/activation, and impaired trafficking of T cells to the tumor mass.
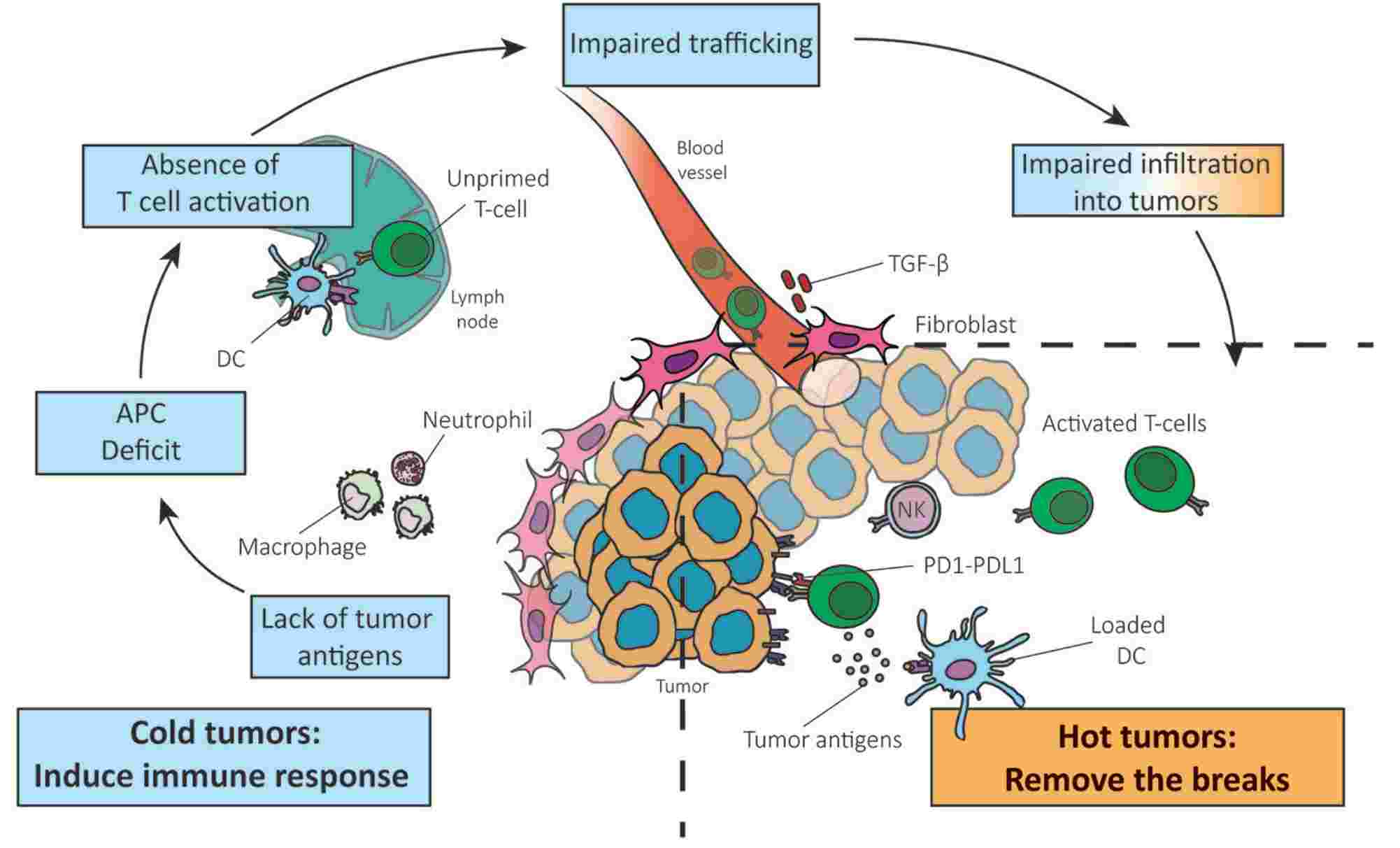 Fig.1. Contributing factors of T cell infiltration declination.1,2
Fig.1. Contributing factors of T cell infiltration declination.1,2
Based on the mechanisms above, several potential paths that target the steps of the cancer immunity cycle are summarized:
Numerous preclinical or clinical trials are underway to apply these strategies in immune therapy. Tumor cell-intrinsic factor CXCL1 has been found to play a predominant role in T cell infiltration in TME, which provided a novel direction of combination with ablation of CXCL1 to promote tumor rejection. As for clinical trials, a STING agonist is being assessed in phase I clinical trial in cutaneous accessible tumors by intra-tumoral administration. In prostate cancer, there are multiple currently ongoing phase II and III clinical trials, providing valuable insights into the strengths and weaknesses of different strategies, and contributing to clarifying the mechanisms of drug resistance that have yet to be surmounted.
Converting a "cold" microenvironment to a "hot" one is a promising strategy to overcome resistance to immune checkpoint therapy. Based on abundant experience and advanced technology platforms, Creative Biolabs provides professional services related to immune checkpoint to assist you in research, including but not limited to:
If you are interested in any one of our services, please do not hesitate to contact us for more detailed inquiries.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2025 Creative Biolabs. All Rights Reserved.