The last decade has seen numerous advances in the outcomes of patients with a range of malignancies due to the development of immunotherapeutic treatments, remarkably immune checkpoint inhibitors (ICIs). Despite proven benefits for many diseases, there remains a significant proportion of patients with these tumors who do not respond or develop resistance. This has led to efforts to enhance ICI responsiveness and overcome ICI resistance.
OVs can target and kill cancer cells while minimizing toxicities to surrounding normal tissues. After infection with these viruses, the local tumor microenvironment is altered with an increase in activated T cells, natural killer (NK) cells, and cytokines. Oncolytic viruses are potentially an effective adjunct to enhance anti-tumor responses in patients that fail to respond to immune checkpoint blockade.
As our understanding of the biological basis of success (or failure) with ICI treatment has grown, the focus has increased on converting inherently immunologically 'cold' tumors to 'hot,' which respond better to ICI in general across tumor types. Amongst the strategies being tested in combination with ICI to turn cold tumors hot, OVs are novel agents with significant promise. The goal of combining OVs and ICIs is to use the viral infection to prime the tumor by altering the local immune microenvironment to one that is more immunogenic, with the understanding that ICI's work best in these hot environments.
As a result, there are now multiple ongoing trials that combine ICI with oncolytic virotherapy. Intratumoral injection of an OV agent in melanoma patients combined with anti-CTLA-4 treatment resulted in a response rate of 50% with a tolerable safety profile in a phase I trial. OV combined with CTLA-4 blockade is studied in a phase II trial and combined with PD-1 blockade in a phase Ib/III trial both in melanoma patients. The latter provided a clinical benefit, and a phase III trial is planned.
A compelling alternative for increased efficacy of ICI is to engineer OVs to encode ICIs, potentially reducing the need for combination therapies. The exquisite selectivity of OVs for cancer cells could lead to localized production of ICIs, which could provide a superior safety profile to systemic administration. This creates an attractive niche for strategies that combine multiple ICIs in a single therapeutic agent. Recent preclinical studies illustrate the feasibility and advantages of such an approach. Using an oncolytic vaccinia virus backbone, OV-encoded PD-1 antibodies can be produced by tumor cells, resulting in markedly better therapeutic efficacy than either OV or anti-PD-1 therapy alone.
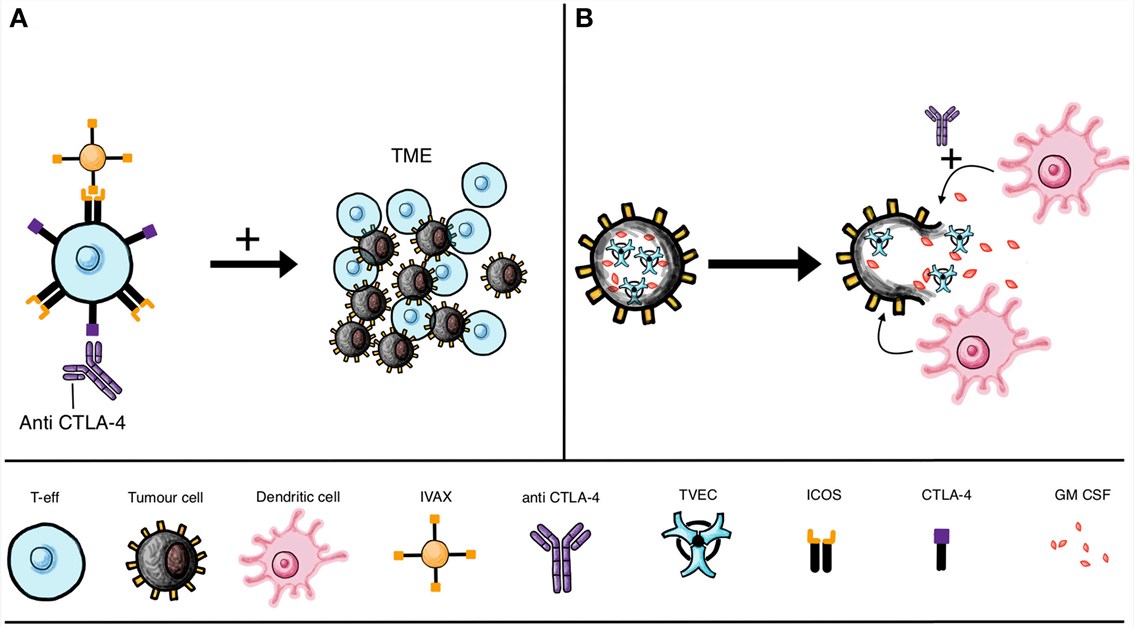 Fig.1. Vaccine and oncolytic viruses combined with checkpoint blockade.1,2
Fig.1. Vaccine and oncolytic viruses combined with checkpoint blockade.1,2
Combining ICI with OVs remains one of the most promising avenues to explore the next step-up in the success of cancer immunotherapy. Based on the most advanced techniques and years of experience in immune checkpoint therapy, Creative Biolabs offers a series of custom services for immune checkpoints, including but not limited to:
Please do not hesitate to contact us for more detailed information.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.