Programmed cell death 1 receptor (PD-1) is a main immune checkpoint molecule that can be widely found on the T cell surface. PD-1 can interact with its ligand, PD-L1, to cause the inhibition of T immune responses. Moreover, pilot studies have demonstrated that some cancer cells can evade immune surveillance through the PD-1/PD-L1 pathway. Therefore, the blocking of the PD-1/PD-L1 pathway has emerged as an effective target for many kinds of disease treatments, especially for various tumors. In recent years, a variety of immune checkpoint inhibitors (ICIs) targeting PD-1 immune checkpoint protein, such as pembrolizumab, have been generated and approved for enhancing the performance of normal immune responses in preventing or treating different types of malignancies.
A2AR, a high-affinity receptor, can be expressed on a number of cell types, such as T cells, macrophages, as well as dendritic cells (DCs). A2AR can interact with extracellular adenosine to inhibit T cell activation. In general, adenosine can be produced by the high expression of CD39 and CD73. It plays an important role in mediating the immunoregulatory activity of regulator T cells by A2AR signaling. Additionally, A2aR activation can suppress the secretion of neutrophils, thereby reducing the inflammatory responses in many human diseases.
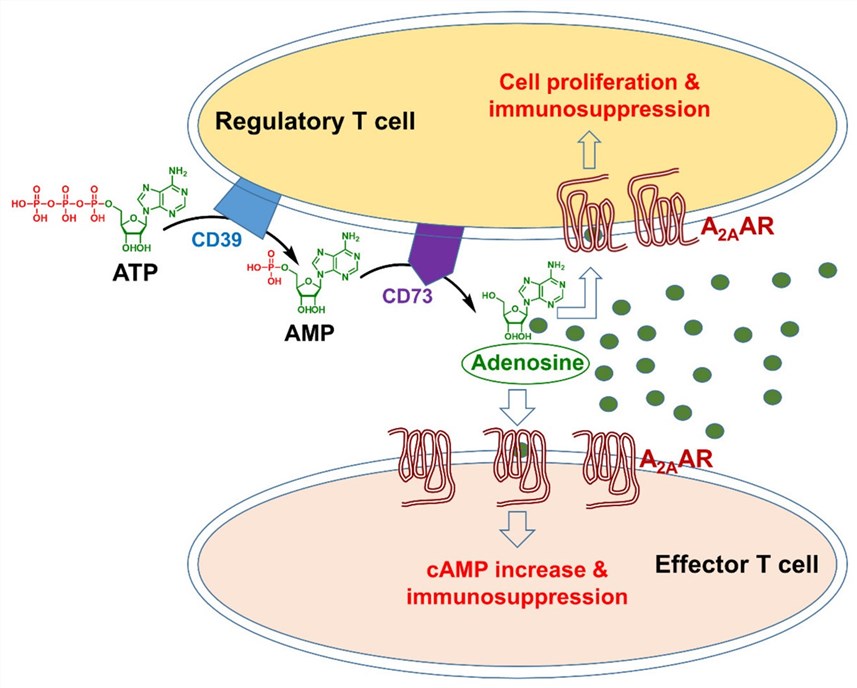 Fig.1. A2AR signaling in the tumor microenvironment.1,2
Fig.1. A2AR signaling in the tumor microenvironment.1,2
Recently, many reports have also revealed that A2AR, an adenosine receptor, is associated with different pathways, like NF-κB pathways, STAT1 pathways, and PPARγ pathways. Furthermore, many types of research have shown that A2AR activation on natural killer (NK) cells can cause reduced anti-tumor cytotoxicity, but blocking the A2AR activation has the potential to increase anti-tumor immunity in different tumor models. As a result, many attempts have been made to develop A2AR antagonists for various disease therapy. For example, several A2AR antagonists have been evaluated and the data have suggested that these antagonists can block the immunosuppressive transcription of T cell or NK cell by inhibiting the accumulation of high-level cyclic adenosine monophosphate (cAMP).
The high expressions of PD-L1 and A2AR have been confirmed in many tumor models, including non-small-cell lung cancer, breast cancer, as well as ovarian cancer. Moreover, a series of PD-1 and A2AR targeted therapies has been established for disease treatments. Among them, PD-1/PD-L1 or A2AR blockades have shown promising data and remarkable therapeutic effects in treating several solid tumors. However, the survival and overall response rates of selected patients are very low, and the resistance mechanism in immune checkpoint therapy is still a major issue for further research.
Currently, the development of combined therapies based on inhibition of PD-1 and A2aR is at the beginning of an exciting time. Till now, many combination strategies for different disease therapy have been broadly studied to further improve positive outcomes and survival in clinical use. For example, a combined therapy based on an A2AR inhibitor and an anti-PD-L1-specific antibody has been designed to further improve the treatment efficacy of checkpoint inhibitors on various disease models.
Creative Biolabs is a leading immune checkpoint therapy development company that provides one-stop services of immune checkpoint molecule discovery, identification, and combined therapy design and evaluation. With our comprehensive immune checkpoint therapy generation platforms, we are ready to help you tackle your toughest research challenge. Please contact us for more detailed information.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.