Semaphorins, which are first identified as axon guidance factors, have a variety of roles in cancer regulation, including angiogenesis, metastasis, and immune evasion. Semaphorins also regulate immune responses between tumor cells and tumor-infiltrating immune cells in the tumor microenvironment (TME) to promote tumor progression.
Semaphorins are a transmembrane and soluble protein family with over 20 different types, which can be classified into classes 3-7: class 3 semaphorins are secreted proteins, while the others are membrane-bound proteins. Under certain conditions, class 4 semaphorins can be cleaved into soluble forms via proteolytic cleavage.
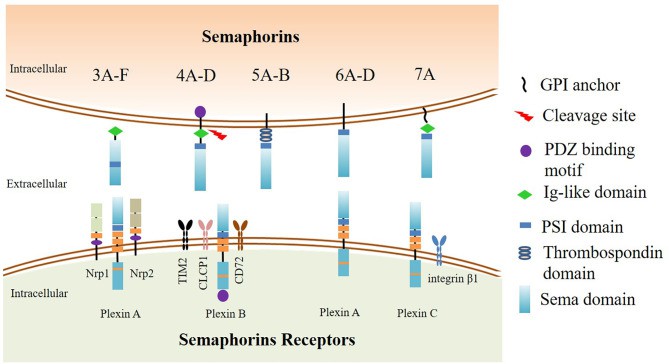 Fig.1. Semaphorins and their receptors: categorization and structure.1,2
Fig.1. Semaphorins and their receptors: categorization and structure.1,2
Semaphorin4D (SEMA4D, also called CD100), a transmembrane molecule of the class-4 semaphorin subfamily, is the first semaphorin to be linked to an immunological response. SEMA4D primarily affects immune cell activity via its receptors plexin and CD72. SEMA4D can promote tumor cell invasion and migration and contributes to tumor angiogenesis via many pathways. Furthermore, SEMA4D plays a complex role in TME because it affects a diverse population of immune cells and contributes to both stimulatory and inhibitory immunological responses.
Sema4D and CD68 (TAMs marker) expression are shown to be significantly higher in gastric tumor tissues than in neighboring normal tissues and to be connected with histological differentiation type, TNM stage, and lymphatic metastasis. TAMs increase SEMA4D expression on cells and accelerate invasion and metastasis in vitro. Anti-SEMA4D treatment reduces tumor mass and enhances survival rates in pancreatic neuroendocrine cancer mice in a short time, but promotes lymph node metastasis, consistent with an increase in TAMs after anti-Sema4D treatment. Macrophages release SDF1 in the presence of anti-SEMA4D antibodies, which promotes tumor cell migration via attaching to the CXCR4 receptor.
SEMA4D has immunoregulatory properties, improves cytotoxic T lymphocytes (CTL) viral clearance in HBV infection, and plays a crucial role in CTL regulation in NSCLC. Blocking Sema4D can boost Treg while decreasing Th1, Th2, and Th17. Furthermore, semaphorin and Nrp-1 expression levels are linked with PD-1 expression levels. Thus, blocking semaphorins, Nrp-1, and PD-1 at the same time may modify CTL anti-tumor function and stop tumor development.
SEMA4D MDSCs are important immunosuppressive cells in head and neck squamous cell carcinomas (HNSCCs). Blocking SEMA4D improved ICI therapeutic responses in HNSCC patients by suppressing PMN-MDSC infiltration by lowering MAPK-dependent chemokine production.
Immune evasion is one of the characteristics of cancer and one of the primary causes of patients' poor prognosis. Semaphorins have become tumor prognostic indicators and therapeutic targets due to their involvement in tumor evasion and progression and disorders in different types of cancers. Semaphorins can serve as attractants, capturing inflammatory cells to the TME. SEMA4D mAb has been discovered to improve immune-checkpoint blockade (ICB) responses. In Murine oral cancer-1 tumors, treatment with SEMA4D mAb prevents PMN-MDSC recruitment by lowering MAPK-dependent chemokine synthesis by tumor cells.
Combination immunotherapy is an effective strategy to remodel TME and boost treatment impact. When used in conjunction with CTLA-4 or PD-1 suppression, SEMA4D mAb increases tumor rejection or reduces tumor progression, contributing to extended life with either treatment. According to recent research, the combination of IgG mAb targeting SEMA4D and PD-L1 inhibitor has been evaluated as a safe and tolerable synthetic treatment in phase II clinical trials in immunotherapy-resistant NSCLC patients.
According to a recent study, semaphorin-based medications with particular antibodies can be delivered to TME via sophisticated nanoparticle or exosome drug delivery systems, potentially alleviating deleterious effects. Adoptive transfer immunotherapy of CTL and NK cells, as well as DC-based vaccines genetically modified with tumor cell semaphorins or receptors, can serve as a viable cancer therapeutic strategy.
Creative Biolabs, as a pioneer and indisputable global leader in drug development, is skilled at implementing advanced custom solutions to meet our customers' diverse project expectations. Creative Biolabs offers a variety of specialized services for the immunological checkpoint molecule semaphorins/SEMA4D, including but not limited to:
Please do not hesitate to contact us if we can be of assistance.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.