Immune Checkpoints of NK Cell
While the immune checkpoints field has predominantly focused on T cell checkpoints, the significance of immune checkpoints in natural killer (NK) cell biology is gaining recognition. In recent years, the field of NK cell biology has witnessed remarkable progress, shedding light on the diverse repertoire of immune checkpoints that govern NK cell function.
Creative Biolabs provides our customers with knowledge sharing in the realm of emerging immune checkpoints in NK cell biology, exploring their roles, regulation, and potential therapeutic implications.
Role of NK Cell Immune Checkpoints in Tumor Immune Evasion
Cancer cells employ various mechanisms to evade immune surveillance. Immune evasion involving NK cells involves several mechanisms.
-
The upregulation of immune checkpoint molecules that inhibit NK cell activity. The inhibitory receptor/ligand pairs, such as killer cell immunoglobulin-like receptors (KIRs) and their HLA class I ligands, play a crucial role in modulating NK cell responses to tumors.
-
Tumor cells or other components of the tumor microenvironment such as TGF-β, IL-6, IL-10, tryptophan catabolites, PGE2, DKK2, IDO, soluble HLA-G, soluble NKG2D ligand, can reduce NK cell activation, cytotoxicity, IFNγ production, and the expression and activation of its activating receptors.
Classical NK Cell Inhibitory Receptors
Inhibitory receptors such as KIRs and CD94/NKG2A have been recognized as the primary immune checkpoints governing NK cell activity. These receptors recognize specific ligands on target cells, triggering inhibitory signals that prevent NK cell activation. Dysregulation or downregulation of inhibitory receptor expression on NK cells can lead to impaired immune surveillance and disease progression.
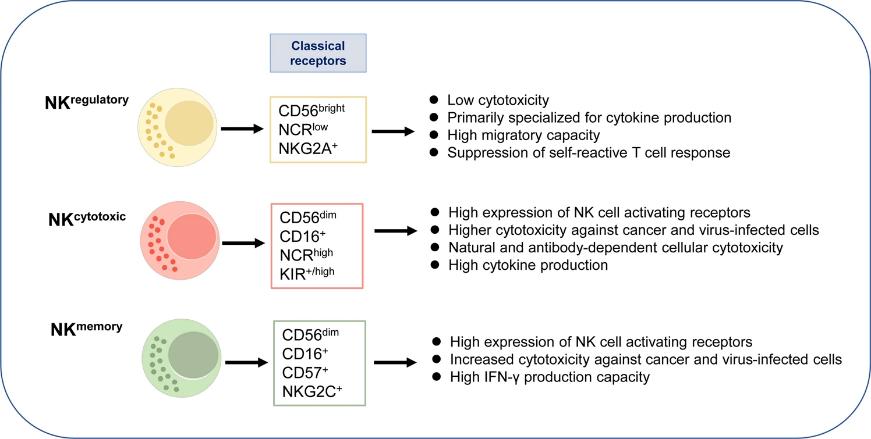 Fig.1. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy.1,2
Fig.1. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy.1,2
-
The KIR family (also known as CD158) is a diverse and polymorphic subtype of NK cell receptors, including inhibitory and activating KIRs, each of which recognizes a specific HLA class I homologue (HLA-A, -B or -C) as a ligand.
-
NKG2A (also known as CD159) and CD94 are heterodimeric inhibitory receptors of the C-type lectin family that recognize the nonclassical MHC-I molecule HLA-E as a ligand. CD94-NKG2A and its HLA-E ligands are non-polymorphic.
Emerging Immune Checkpoints in NK Cell
Recent studies have uncovered an expanding repertoire of immune checkpoints that modulate NK cell functions. These emerging checkpoints include both cell surface receptors and intracellular signaling molecules, offering novel therapeutic opportunities in the field of NK cell immunotherapy.
-
One class of emerging immune checkpoints on NK cells comprises a diverse group of receptors that fine-tune NK cell responses. Among them, TIGIT and CD96 have gained considerable attention. These receptors exert inhibitory effects on NK cell activity by engaging their respective ligands. Additionally, these checkpoint receptors can modulate NK cell functions through crosstalk with other signaling pathways, including T cell checkpoints.
-
Apart from cell surface receptors, intracellular signaling checkpoints also play a crucial role in regulating NK cell-mediated immunity. Various signaling molecules, such as SHP-1, SHIP-1, and DAP12, function as negative regulators of NK cell activation. These molecules, when dysregulated or impaired, can disrupt the delicate balance between activating and inhibitory signals, resulting in altered NK cell functions.
Understanding the complex interplay of emerging immune checkpoints in NK cell biology opens new avenues for therapeutic interventions in cancer and other diseases. At Creative Biolabs, we are committed to unraveling the intricacies of NK cell biology and leveraging emerging immune checkpoints to develop innovative immunotherapeutic strategies.
References
-
Cao, Yuqing, et al. "Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy." Signal transduction and targeted therapy 5.1 (2020): 250.
-
Under Open Access license CC BY 4.0, without modification.
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
 Fig.1. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy.1,2
Fig.1. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy.1,2