The intricacies of immune checkpoint regulation extend far beyond their individual functions. Recent breakthroughs have illuminated the phenomenon of mutual regulation, a concept that reveals the dynamic interplay between these checkpoints. CTLA-4, for instance, not only fine-tunes T-cell activation but also influences the expression and function of PD-1, and vice versa. This cross-talk between checkpoints adds layers of complexity to our understanding of immune modulation.
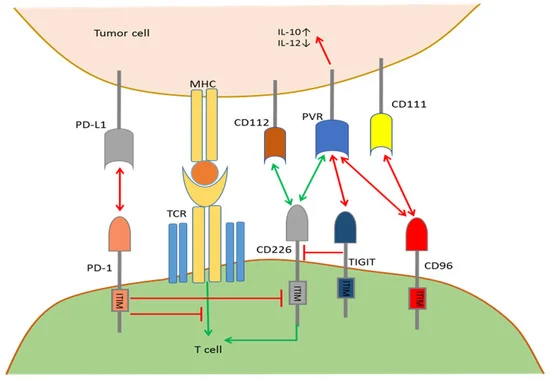 Fig.1. Mutual regulation by the PD-1/CD226/TIGIT/CD96 pathways.1,2
Fig.1. Mutual regulation by the PD-1/CD226/TIGIT/CD96 pathways.1,2
PD-1 is primarily expressed on activated T cells, B cells, and myeloid cells, while CTLA-4 predominantly resides in regulatory T cells (Tregs). Their signaling pathways converge on dampening T cell activity, but they employ distinct mechanisms.
The mutual regulation between PD-1 and CTLA-4 is a dynamic process.
TIM-3 is expressed on exhausted T cells and plays a pivotal role in dampening their function. PD-1 and TIM-3 often co-exist on exhausted T cells, and their interaction can have profound implications for immune responses in cancer.
Recent studies have unveiled a compelling connection between PD-1 and TIM-3.
LAG-3 is expressed on activated T cells and regulatory T cells and is involved in modulating T cell responses. The mutual regulation of LAG-3 and PD-1 is a topic of growing interest in the field of immunotherapy.
As researchers delve deeper into the intricacies of these interactions, we can expect the development of tailored combination therapies that harness the power of mutual regulation to enhance antitumor immunity.
At Creative Biolabs, we remain dedicated to advancing the field of immunotherapy through cutting-edge research and innovative solutions. Stay tuned for more updates on the exciting developments in the world of immune checkpoint modulation.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.