Probiotics are live microorganisms that, when consumed in sufficient amounts, can provide a variety of health benefits. Traditionally, probiotics have been associated with gut health and digestion. However, recent advances have revealed their wide-ranging potential to influence the immune system.
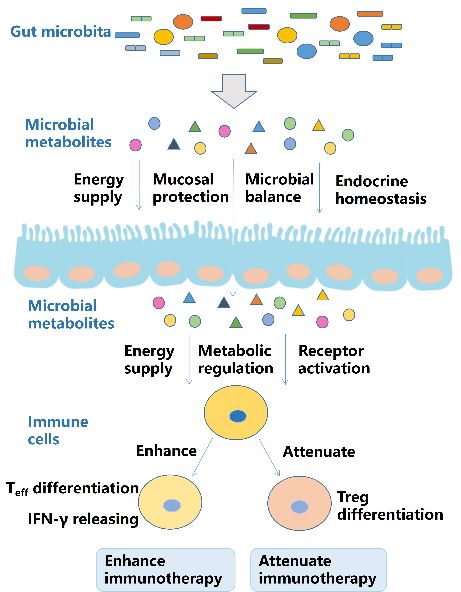 Fig.1. Role of microbial metabolites on the immune system.1,2
Fig.1. Role of microbial metabolites on the immune system.1,2
Probiotic metabolites are metabolic by-products of probiotic microorganisms. In recent years, these small molecules have attracted much attention for their ability to modulate immune responses. Their role in promoting immune checkpoints for anti-tumor therapy is an exciting avenue to explore.
One such metabolite is short-chain fatty acids (SCFA), which are produced by certain probiotics in the gut.
SCFA, including butyrate, acetate, and propionate, have been shown to have a profound effect on the immune system. The potential to unlock these metabolites may be key to enhancing anti-tumor immune responses.
The relationship between probiotic metabolites and immune checkpoint regulation is a promising area of research. Several studies have shown that probiotic metabolites, particularly SCFA, can influence the expression of immune checkpoint proteins such as PD-1 and PD-L1.
Research on probiotic metabolism for immune checkpoint therapy is ongoing, and we give some relevant examples of what is being studied. You can also check the latest scientific literature for the most up-to-date information.
The symbiotic relationship between probiotic metabolites and immune checkpoints offers a compelling avenue for the development of novel and effective cancer therapies. Our team of dedicated scientists is at the forefront of unraveling the complex interactions between probiotic metabolites and immune checkpoints. Through rigorous experimentation and extensive research, Creative Biolabs is working tirelessly to develop new therapies.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.