As we venture deeper into the realm of immune regulation, one intriguing aspect that has recently captured the attention of the scientific community is the glycosylation of immune checkpoints. In the context of immune checkpoints, the addition of sugars to these key regulatory molecules has been shown to significantly influence their localization, interaction partners, and ultimately, their immune-modulating capabilities.
Glycosylation adds a layer of complexity to the immune checkpoint landscape, introducing structural diversity and fine-tuning of their immunomodulatory activities.
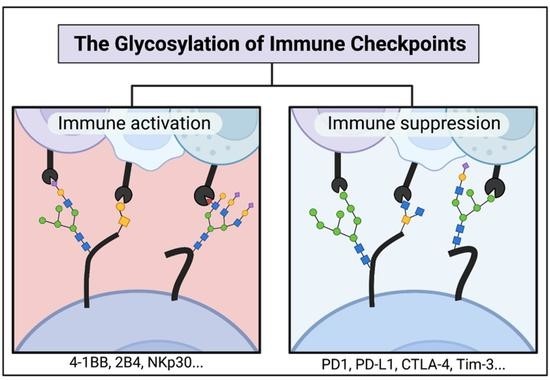 Fig.1. The glycosylation of immune checkpoints.1,2
Fig.1. The glycosylation of immune checkpoints.1,2
One of the most intriguing aspects of glycosylation is its diversity and heterogeneity.
As the significance of glycosylation in immune checkpoint biology continues to be unveiled, researchers are harnessing this knowledge to design innovative therapeutic strategies. Glycoengineering, the manipulation of glycosylation patterns, emerges as a promising avenue to fine-tune the activity of immune checkpoints.
By selectively removing specific glycans or shielding them with chemical modifications, this approach can increase the accessibility of key binding sites, enhance checkpoint-receptor interactions, and potentiate downstream signaling, thus augmenting the immune response against tumors.
Conversely, introducing novel glycans to immune checkpoints can expand their ligand binding repertoire, broadening the range of interactions and potentially engaging new signaling pathways. This strategy can amplify the anti-tumor immune response by unlocking previously untapped immune regulation mechanisms.
By engineering site-specific glycosylation, researchers can tailor the immunomodulatory properties of checkpoints, enhancing anti-tumor activity while minimizing unwanted immune-related adverse events.
Modulating the expression or activity of these enzymes can alter the glycosylation landscape of immune checkpoints, leading to improved therapeutic outcomes.
By strategically pairing glycoengineered immune checkpoint inhibitors with other immunotherapies, such as cancer vaccines or adoptive T-cell therapies, researchers aim to unleash the full potential of the immune system in combating cancer.
At Creative Biolabs, we remain committed to unraveling the mysteries of glycosylation and its impact on immune checkpoints, opening new pathways for researchers to develop safer and more effective therapies.
Reference
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.