Immune checkpoints are negative feedback mechanisms of the immune system that, when activated, protect normal cells from T cell-mediated cytotoxicity and maintain immune homeostasis. Well-known checkpoints (PD-1, CTLA-4, TIM3, LAG3, etc.) are expressed on the surface of the cell membrane. In addition to this, a number of intracellular proteins are involved in negative feedback loops downstream of the TCR and can be considered as intracellular immune checkpoints, several of which are currently in the clinical research stage.
Creative Biolabs introduces some intracellular immune checkpoints and gives some relevant information about their development and application.
PD-1 inhibits T cell activation by recruiting the phosphatase SHP-2. However, T cell-specific deletion of SHP2 in mice did not improve anti-tumor immunity. It was later found that in the absence of SHP-2, PD-1 recruited SHP-1 to maintain this function. Thus, blocking both SHP-1 and SHP-2 is necessary to improve the TCR signaling pathway.
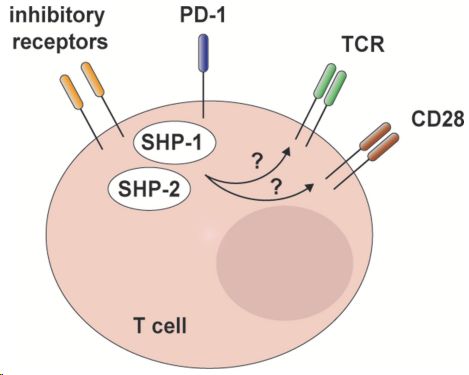 Fig.1. SHP-2 and redundant mechanisms in T cell inhibitory receptor signaling.1,2
Fig.1. SHP-2 and redundant mechanisms in T cell inhibitory receptor signaling.1,2
In the bone marrow, GM-CSF induces phosphorylation of PD-1 and recruitment of PD-1-SHP-2 to the GM-CSF receptor. Deletion of SHP-2 or PD-1 enhances GM-CSF-mediated phosphorylation of the transcription factors HOXA10 and IRF8, which regulate medullary differentiation and mono-moDC spectrum differentiation, respectively.
There are many ongoing clinical studies of anti-PD-1 antibodies in combination with SHP-2 inhibitors. SHP2 inhibitor in combination with anti-PD-1 antibody in patients with advanced non-small cell lung cancer.
CBL proteins have a ring-finger catalytic structural domain that is responsible for protein ubiquitination and sequential degradation of target proteins. CBL proteins lead to the degradation of multiple targets, thereby down-regulating the TCR signaling cascade. CBL-b targets the regulatory subunit of PI3K, p85, which reduces the activity of PI3K and interferes with its ability to activate different signaling pathways.
CBL-b is involved in co-stimulatory signaling that regulates CD28 or inhibits the receptors CTLA-4 and PD-1. Loss of CBL-b on the TCR leads to Akt/Erk phosphorylation, proliferation, activation, cytokine production (IFN γ, TNFα, IL-2), and cytolytic capacity (granzyme B).
Several clinical trials to inhibit CBL-b in T cells are ongoing.
CISH, a member of the Suppressor of Cytokine Signaling (SOCS) family of proteins, negatively regulates the CD8+ T cell signaling pathway. CD8+ T lymphocytes from defective mice showed improved response to TCR signaling and increased expression of effector function-related genes (Il2, Prf1, GrzmB, Eomes, Tbx21, c-Myc, and Bcl2l).
Clinical trials are underway to knock down CISH in TILs by CRISPR-Cas9 for tumor therapy.
At Creative Biolabs, we are at the forefront of these advances, working to immune checkpoint targeted small molecule development. Please do not hesitate to contact us with your particular needs.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.