Mouse tumor cell transplant models are widely applied by the pharmaceutical industry to develop chemotherapies and targeted therapies for their advantages of low cost, reproducibility, synchronous growth of tumors, and easiness for longitudinal tracking of tumor growth. Mouse tumor cell transplant models consist of syngeneic tumor mouse models and xenograft tumor mouse models.
The syngeneic tumor mouse models can be divided into CDA models (cell line-derived allograft models, created by inoculating mouse-derived tumor cell lines into immunocompetent inbred mice) and MDA models (mouse-derived allograft models, created by inoculating mouse-derived tumor tissue into immunocompetent inbred mice). The identical genetic background prevents rapid allogenic responses against transplanted tumor cells but still allows the host to mount specific immune responses against tumor antigens for it still has a completeness of the immune system.
The syngeneic tumor mouse models are widely used to study the immunotherapy of tumors and the preclinical development of checkpoint therapies. Previous studies have already identified some actionable immune checkpoints like CTLA-4 and PD-1 through the employment of syngeneic mouse models. Nowadays, increasing technical methods like X-ray micro-computed tomography (micro-CT), ultrasound imaging, positron emission tomography (PET), bioluminescence, and magnetic resonance imaging (MRI) are applied in tumor cell transplant models. However, although the syngeneic tumor mouse model has many advantages, it still has some shortcomings that restrict its further application such as the high mortality of the injected genetically homogeneous cancer cells, the lack of the features of the focal multi-step carcinogenesis that occurs in natural circumstances, human-mouse species differences, and, last, limited cancer types.
The xenograft tumor models are also divided into two types. The first one is patient-derived xenograft models (PDX) which is established by directly transplanting the fresh tumor tissue collected from patients into the immunodeficient mice. The second one is the cell line-derived xenograft model (CDX) which is constructed by transplanting human tumor cell lines cultured in vitro into immunodeficient mice. As a classic in vivo experimental method, CDX is in great demand in oncology research and anti-tumor drug development for the numerous advantages of low cost in terms of time and money, high reproducibility, and high tumorigenesis rate. Similar to syngeneic models transplanted with mouse cell lines, CDX models failed to predict the efficacy of many drug candidates for targeted therapies due to the lack of tumor etiology and heterogeneity. As an improvement to CDX models, PDX mice received surgically derived human tumor specimens subcutaneously or orthotopically are generated with intact human tissue microenvironment and diverse tumor heterogeneity. These models successfully predicted the drug responses in humans where CDX models failed. PDX models hold great promise for therapeutic evaluation, restricted patient sample access, low take rate for certain tumor types, decreased prediction power in high passages, and missing host immunity remain critical issues to be overcome.
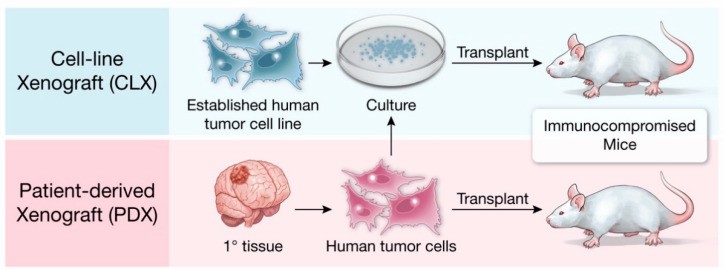 Fig.1. Methods to generate xenograft tumor mouse models.1,2
Fig.1. Methods to generate xenograft tumor mouse models.1,2
As a global biotechnology services company, Creative Biolabs has been at the forefront of biological research. We have sufficient expertise, a team of experts working in the field of tumor immune checkpoint and drug development for a long time, and reliable products. If you need some services or products related to tumor immune checkpoint and tumor cell transplant mouse model, please do not hesitate to contact us. Our comprehensive services include, but are not limited to:
For more information, please click on our Services Overview.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.