Creative Biolabs offers tumor mutational burden (TMB) and neoantigen load as predictive biomarkers. Elevated TMB has established associations with favorable responses to immune checkpoint therapy (ICT) across various cancer types, notably melanoma and non-small cell lung cancer (NSCLC). High TMB is believed to contribute to the generation of neoantigens originating from variations within the coding sequences of expressed proteins within the tumor cells, which can be recognized by the immune system.
One promising avenue for enhancing antigen-specific T-cell responses involves combining ICT with personalized neoantigen vaccines. These vaccines have demonstrated the ability to modulate T-cell activity within the tumor microenvironment (TME), leading to improved responses to ICT. As vaccine therapies continue to evolve, encompassing optimal formulations, adjuvants, and delivery methods capable of eliciting robust antitumor immune responses, the harnessing of tumor mutations to develop neoantigen vaccines in conjunction with ICT may hold the key to successful therapeutic strategies.
| As tumors progress, they accumulate mutations, a subset of which generate neoantigens, impacting patients' immune checkpoint inhibitor responses. Neoantigens represent emerging predictive biomarkers. Notably, subclonal neoantigens induced by cytotoxic chemotherapy, leading to heightened mutational burden, predominated in specific non-responsive cases. These findings underscore the potential influence of neoantigen heterogeneity on immune surveillance and advocate therapeutic strategies aimed at clonal neoantigens. | |
| TMB and neoantigen load exhibit significant variability both within and across various tumor types, with melanomas, lung cancers, and bladder cancers displaying notably elevated TMB levels compared to other tumor types. Additionally, the mutational landscape can exhibit heterogeneity within individual tumors and undergo changes during tumor progression or subsequent to treatment interventions. The clinical efficacy of immunotherapy has been substantiated in patients with dMMR and MSI-high tumors, further affirming that TMB, serving as a surrogate marker for genomic instability and tumor neoantigens, remains a pertinent biomarker for predicting immunotherapeutic responses. | |
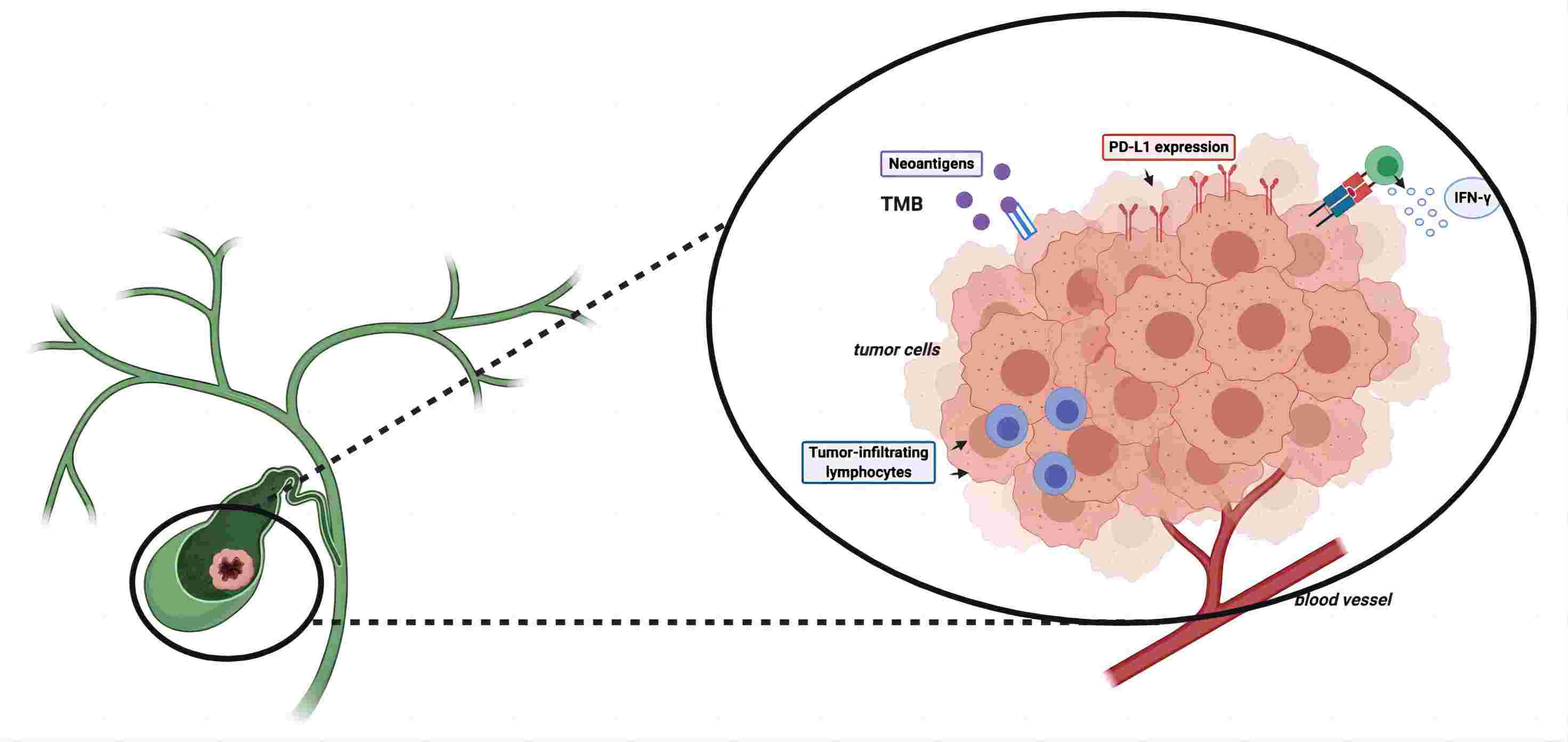
|
Fig.1. Potential immune checkpoint inhibitor (ICI) response biomarkers.1,2 |
Creative Biolabs provides an extensive range of tailored services in the realm of immune checkpoints in conjunction with predictive biomarkers, encompassing, but not limited to: Custom Immune Checkpoint Protein Development, Antibody Development for Immune Checkpoints, Immune Checkpoint Targeted Peptide Development, Immune Checkpoint Targeted Small Molecule Drug Development, Biomarker Development for Immune Checkpoint Inhibitor (ICI), etc. For a comprehensive overview, please do not hesitate to contact us.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.