Creative Biolabs is pleased to introduce the crucial role that PD-L1 plays in guiding cancer intervention decisions and serving as a biomarker of biological response to maximize the potential benefits of immune checkpoint blockade while avoiding unnecessary toxicity.
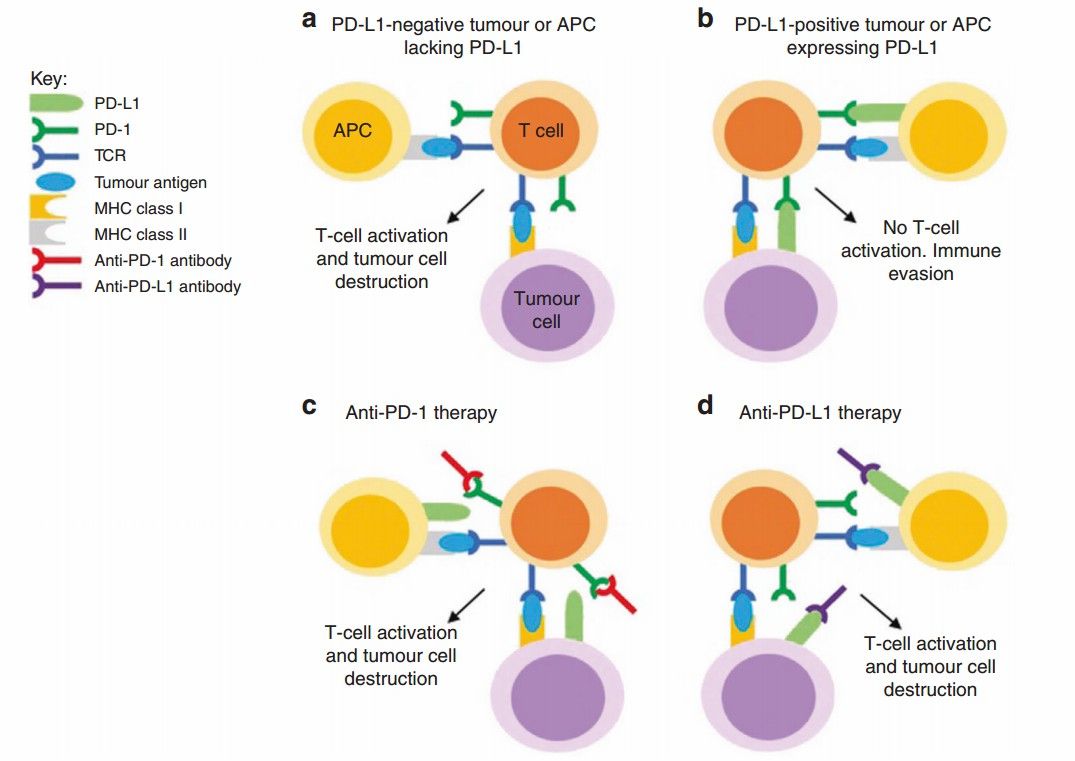 Fig.1. Anti-PD-1 and anti-PD-L1 antibodies.1,2
Fig.1. Anti-PD-1 and anti-PD-L1 antibodies.1,2
PD-L1 expression has logically become a biomarker for measuring the existence and distribution of molecular targets analyzed by therapeutic antibodies. In fact, several studies have shown that PD-L1-positive tumor patients exhibit better ICB clinical benefit rates and tumor response rates across multiple cancer types and have been approved as companion diagnostic indicators for several indications.
Predicting potential ICB responses through PD-L1 has shown significant variability in different research or drug conditions, even displaying contradictory results. However, more promising approaches are currently being investigated, including:
Title: Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer.
Research Objective:
To evaluate the anti-tumor activity and safety of specific blockade of PD-1 antibodies and predict possible immune resistance.
Methodology:
Treatment with anti-PD-1 antibodies was administered to patients with non-small cell lung cancer, renal cell carcinoma, colorectal cancer, advanced melanoma, and prostate cancer, and their effectiveness was evaluated after a treatment cycle.
Research Findings:
In a total of 236 patients, complete or partial relief of symptoms was observed in non-small cell lung cancer, renal cell carcinoma, and melanoma. The existing adverse conditions did not affect the effects of treatment. This proved that there might be a correlation between PD-1 expression levels on tumor cells and the inhibitory effect of tumor immune checkpoints.
Here at Creative Biolabs, we aim to assist you in exploring more possibilities and providing robust support for the development of accurate and feasible predictions of ICB effects and tumor intervention strategies through our comprehensive immune checkpoint services and technical support. Contact us now to explore the full potential of PD-L1 in immunotherapy.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.