The tertiary lymphoid structure (TLS) has emerged as a prominent biomarker for assessing the response of various tumor structures to immune checkpoint therapy. Here at Creative Biolabs, we present crucial information about the role of TLS in tumor immunology and its significance as an immune intervention predictor biomarker.
TLS represents an anatomical entity akin to secondary lymphoid structures, comprising tumor-infiltrating lymphocytes, including T-cells, B-cells, high endothelial venules, and DC cells. TLS serves to modulate the immune microenvironment by augmenting local immune responses and recruiting circulating immune cells, thus facilitating tumor clearance by means of infection control or rejection reactions.
The potential mechanisms through which TLS acts against cancer include:
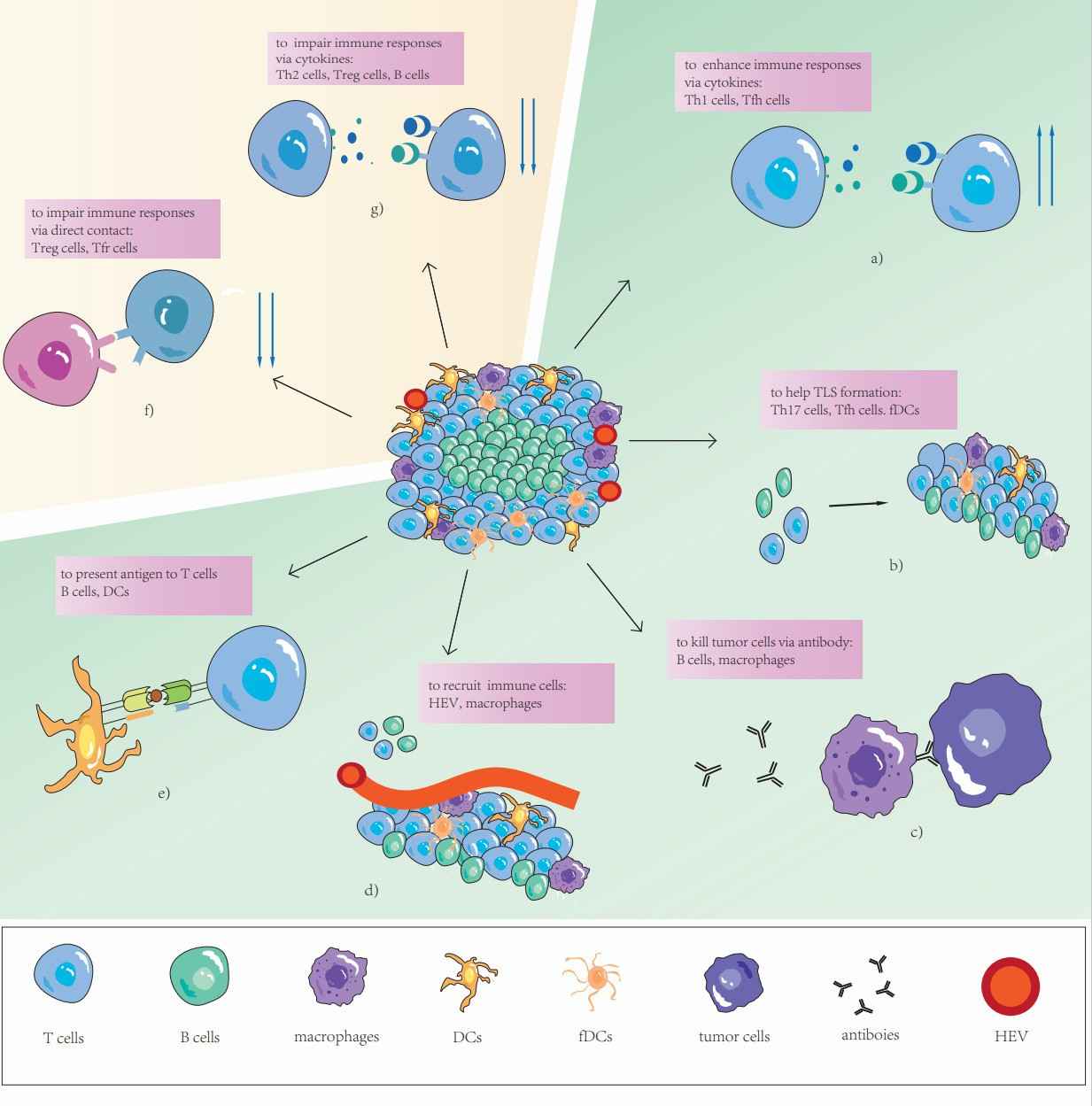 Fig.1. TLS and its components.1,3
Fig.1. TLS and its components.1,3
Substantial fundamental research has unequivocally demonstrated the association between TLS existence and favorable outcomes in PD-1-based or PD-1/CTLA-4 combination blockade therapies, validating the predictive role of TLS on the biology behaviors, treatment methods, and prognosis of various tumors:
However, it is essential to note that TLS is a multi-cell dynamic structure with significant heterogeneity, and different molecular markers or datasets need to be considered for evaluation in different cancer types.
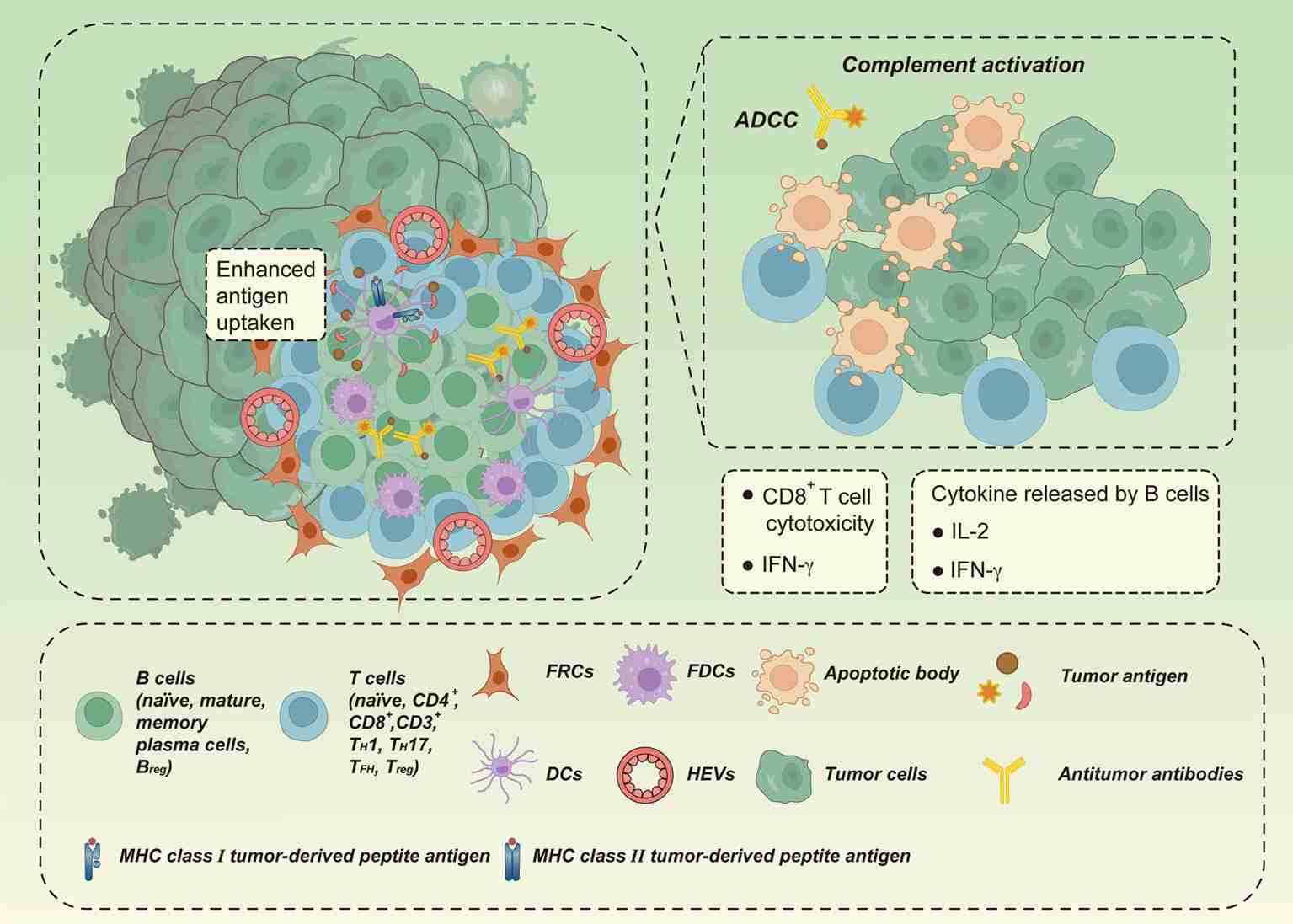 Fig.2. Antitumor effect of TLS.2,3
Fig.2. Antitumor effect of TLS.2,3
Due to the extensive participation of TLS in anti-tumor responses, inducing TLS formation is considered a promising tumor intervention strategy:
Although inducing or enhancing TLS is beneficial for tumor control, the application of these strategies may also promote self-reactive immune responses, requiring additional scrutiny and research.
At Creative Biolabs, we transcend mere theoretical support and offer a comprehensive range of advanced services in the realm of immune checkpoint. From biomarker identification and validation to preclinical investigations and clinical trial assistance, our multidimensional approach ensures unparalleled support throughout the entire process. Contact us now to explore the full potential of immune checkpoint immunotherapy.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.