Immune checkpoints (ICPs) are a group of molecules found on the surface of Treg cells and other immune cells that play an important role in maintaining autoimmune homeostasis and immunological tolerance due to their flexible modulation of the length and severity of physiological immune responses. The application of immune checkpoint therapy in Type 1 Diabetes (T1D) is an area of active research and holds promise for future treatments.
Considered a significant risk to health and survival, T1D is a heterogeneous autoimmune disease driven by T cells, which is mainly due to an immune-mediated attack on the islet beta cells, leading to a loss of insulin production, exogenous insulin dependency, and subsequent high blood sugar levels.
PD-1, which is normally expressed on chronically activated T lymphocytes in peripheral organs, is also found on pancreatic islet cells. By attaching to the ligand PD-L1 or PD-L2, PD-1 sends negative signals to T cells, increasing immune response inhibition. In mice, boosting PD-1/PD-L1 expression or utilizing medications to repair the PD-1/PD-L1 pathway altered the course of diabetes. In normal populations, the surface of islet cells expresses PD-1 for self-protection, while the surface of T cells allows PD-1-expressing islet B cells to circumvent the immune response via ICP. Increasing ICP expression to shield pancreatic islet-cells from T-cell attack has the potential to reverse early-onset T1D or improve prognosis. In bioengineered-cells, ICPs can also protect human islet-like organ transplants from T-cell attack, establish antigen-specific immunological tolerance, and reverse early-onset hyperglycemia.
In that way, pancreatic islet-cells attempt to suppress the immunological response by upregulating PDL1, thereby preventing further tissue damage. During islet inflammation, PDL1 expression in pancreatic islet B cells is greatly increased, which is considered to be triggered by type I and type II IFNs. Furthermore, interferon is a key regulator of PDL1 expression in human pancreatic cells.
Immune checkpoint molecules exhibit the potential as a therapeutic maneuver for T1D control. Specific immune checkpoint mechanisms also participate in pathological processes of T1D. A recent study showed that higher levels of circulating immune checkpoint molecules, especially CD137/4-1BB and PD-1, may serve as prognostic biomarkers for new-onsets T1D and risk factors for the growth of an additional autoimmune disease.
Sub-immunogenic immunization with a strong agonistic insulin mimotope successfully inhibits effector T cells and upregulates Foxp3, CTLA4, IL-2R, and TIGIT expression, providing a potential novel therapeutic target for T1D islet autoimmunity prevention. In autoimmune diabetic animal models, an anti-PD-1 immunotoxin specifically targeting and depleting PD-1-expressing cells delays disease onset. In new hyperglycemic NOD mice, genetically engineered PD-L1-overexpressing platelets inhibit autoreactive pancreatic T-cell activity and restore diabetes. Aside from CTLA-4 and PD-1, the next wave of co-inhibitory immune checkpoint receptor targets, such as LAG-3, TIM-3, and TIGIT, is gaining traction in clinical trials.
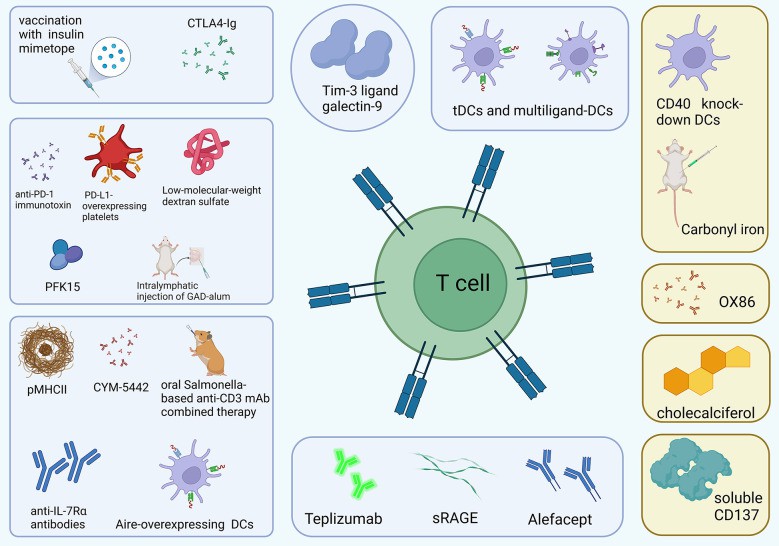 Fig 1 T1D therapy works by controlling T cells.1,2
Fig 1 T1D therapy works by controlling T cells.1,2
Creative Biolabs offers a series of custom services for developing immune checkpoint therapy in T1D. If you require any additional information, please do not hesitate to contact us.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.