Creative Biolabs aims to leverage our expertise in immunology to provide you with comprehensive knowledge of immune checkpoints related to systemic sclerosis (SSc), helping you develop promising SSc intervention strategies.
SSc is an autoimmune T cell disease characterized by pathological fibrosis in the skin and visceral organs. The complex and dysregulated pathological mechanisms of SSc are not yet fully understood. However, increasing evidence suggests that the interaction between the immune system and fibroblasts in the early stages of the disease is the primary driver of fibrosis progression.
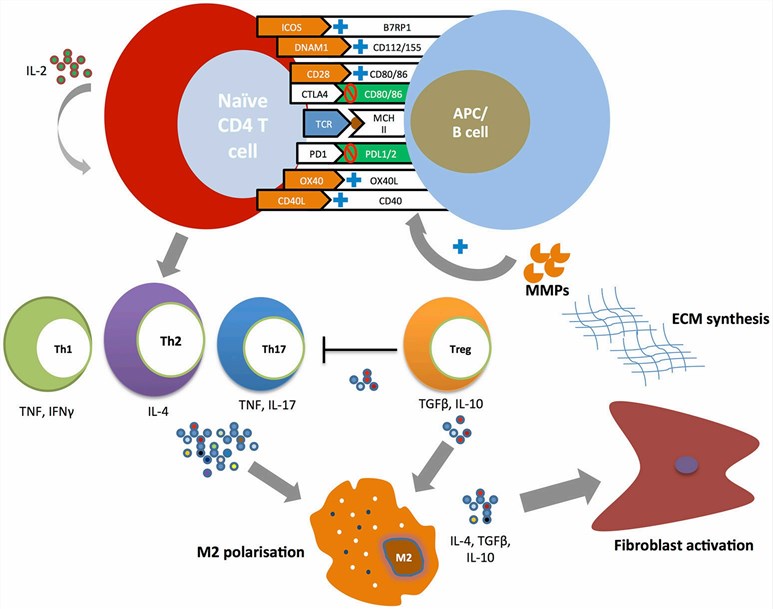 Fig.1. Costimulatory pathways in the pathogenesis of SSc.1,2
Fig.1. Costimulatory pathways in the pathogenesis of SSc.1,2
Immune checkpoint-related co-stimulatory pathways play a role in suppressing adaptive immune responses, and abnormalities in their function and expression have been reported to be closely associated with the pathological progression of SSc.
| Immune Checkpoint | Stimulatory Signal Type | Pathological Association | Possible Mechanisms |
| CD40-CD157 | Positive |
Increased CD40 expression in activated CD4+ T cells and serum/skin fibroblasts of SSc patients Soluble CD40L levels associate with vascular complications |
|
| CD28-CD80/86 | Positive | Increased soluble CD28 levels in SSc patients inhibit T cell response | Activation of T cells and increased pro-inflammatory/pro-fibrotic cytokines |
| OX40-OX40L | Positive | Elevated serum levels of OX40L in SSc patients associate with worsened fibrosis | Directly mediating pathological activation of dermal fibroblasts and promoting fibrosis via matrix metalloproteinase-mediated fibrosis |
| CTLA-4-CD80/86 | Negative | Serum-soluble CTLA-4 levels directly correlate with disease severity and activity | Abnormal activation of T cells and B cells |
| PD-1-PD-L1/2 | Negative | Soluble PD-1 levels correlate with disease severity | Abnormal activation of T cells and B cells |
Traditional SSc interventions are mostly palliative and cannot effectively reverse the course of the disease. In fact, targeting immune checkpoints for SSc may be a more advantageous strategy: Targeting antigen-specific T cells involved in the disease through immune checkpoint modulation avoids additional systemic immunosuppression and reduces the theoretical risk of infections.
OX40L blockade achieved through gene knockout or monoclonal antibodies has been shown to reduce the infiltration of inflammatory cells into affected tissues in mouse models. This effect is achieved by reducing the levels of NK cells, T cells, B cells, and pro-inflammatory cytokines.
Blocking T cell co-stimulation through soluble inhibitory receptor-Fc fusion protein (CTLA-4-FC) has been proven effective in preventing or eliminating inflammation-driven dermal fibrosis. It also improves gastrointestinal involvement and reduces the release of pro-inflammatory/pro-fibrotic cytokines. This approach has demonstrated potential effectiveness in clinical trials.
Checkpoint molecules such as TIGIT, PD-1, VISTA, and FcγRIIB may represent promising target options for autoimmune diseases. Combination or alternate targeting of different receptors is also being investigated and validated.
At Creative Biolabs, we offer a comprehensive range of services that go beyond theoretical support. We are dedicated to providing you with exceptional support throughout your journey in developing innovative interventions for SSc.
Our commitment to excellence means that we will work closely with you to understand your unique requirements and design a personalized service plan.
Contact us today to discover how our unparalleled services can accelerate your research. Together, let us make significant strides towards conquering systemic sclerosis and improving patient outcomes.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.