Blockade and dysregulation of immune checkpoints can lead to a variety of complex adverse events related to the immune system, including the most devastating systemic lupus erythematosus (SLE). Here, Creative Biolabs is pleased to introduce the intricate and complex connections between immune checkpoint pathways and SLE, and assist you in manipulating and harnessing these immune signaling axes for effective outcomes.
The clinical and pathological causes of SLE are highly complex but are generally attributed to the loss of self-tolerance due to excessive activation of autoreactive T cells, which in turn promotes the overexpression of antibodies and pro-inflammatory cytokines by B cells.
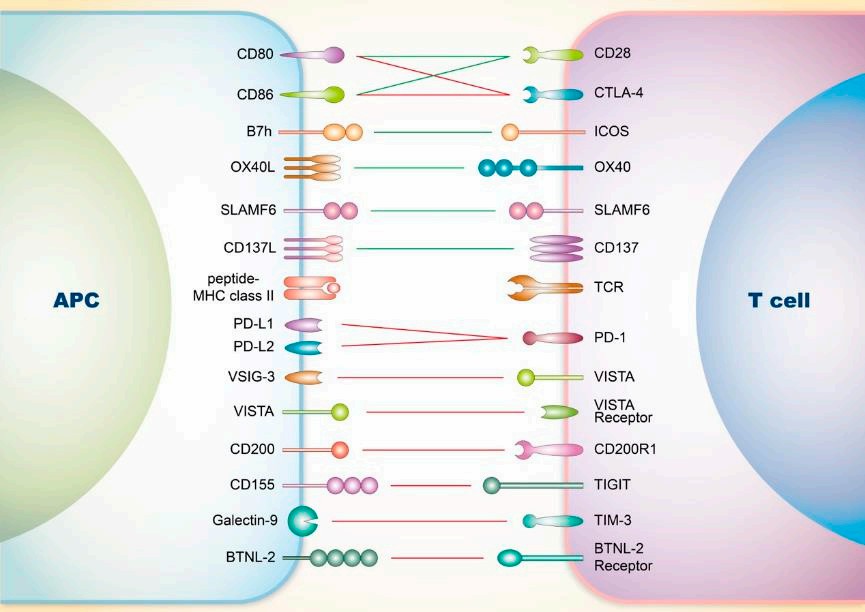 Fig.1. Regulation of the SLE and T cells.1,2
Fig.1. Regulation of the SLE and T cells.1,2
During the pathogenesis of SLE, tolerance breakdown in CD4+ T cells often occurs, with key immune checkpoint molecules on T cells believed to determine their fate.
| Molecule | Ligand/Receptor | Action Mode | Function | SLE Intervention |
| CD80, CD86 | CD28 | Co-stimulation | Full activation of T cells | Anti-CD28 protein has entered phase II clinical trials |
| CD80, CD86 | CTLA-4 | Co-inhibition |
Susceptibility to SLE is associated with CTLA-4 promoter polymorphism Affects TFH and Treg; deficiency leads to production of autoantibodies and non-infectious inflammation |
CTLA-4 fusion protein and CTLA-4-Fc are under development |
| B7h | ICOS | Co-stimulation |
Promotes downstream humoral immunity Inflammation in SLE depends on PI3K-mediated ICOS stimulation |
Targeting ICOS ligand with human antibodies has demonstrated some efficacy and safety |
| OX40L | OX40 | Co-stimulation |
Interaction between OX40 and its ligand stimulates TFH responses and contributes to SLE pathology Expression of OX40 is associated with the severity and susceptibility of SLE |
Anti-OX40 therapy can significantly reduce IL-10 expression and delay the occurrence of severe proteinuria |
| CD137L | CD137(4-1BB) | Co-stimulation | Loss of CD137L worsens renal and cutaneous manifestations of SLE but alleviates neurological damage | Agonistic anti-CD137 mAb can reverse SLE and extend lifespan in mice without inducing immune suppression |
| PD-L1, PDL2 | PD-1 | Co-inhibition |
Deficiency of murine PD-1 leads to lupus-like autoimmune disease Blocking PD-1 affects TFR cell function, thereby alleviating the severity of lupus |
|
| CD200 | CD200R1 | Co-inhibition |
Regulates the inflammatory threshold, Th2 polarization, and immune balance Increased numbers of CD200+ cells and soluble CD200 have been observed in SLE patients |
|
| CD155 | TIGIT | Co-inhibition |
The severity of SLE is associated with the frequency of CD3+CD4+ T cells Activation of the TIGIT pathway significantly downregulates CD4+ cell activity in SLE patients |
Targeted drugs can significantly delay the pathological progression in SLE mice |
Most of the current interventions targeting immune checkpoints in SLE have focused on shared signaling pathways in T cells. Although such approaches have shown promising potential for pathological inhibition, there is still no successful methodological theory.
The production of anti-nuclear self-antibodies by B cells and the breakdown of self-tolerance are considered the core pathological mechanisms in SLE. Several B-cell immune checkpoint molecules have been identified as being associated with SLE.
| Molecule | Ligand/Receptor | Action Mode | Function | SLE Intervention |
| CD154 | CD40 | Co-stimulation | CD40L is ectopically expressed in SLE patients and mice, accompanied by immune complex deposition | Anti-CD40L mAb can block overexpression of CD86 in SLE patients and inhibit the production of anti-DNA antibodies |
| PD-L1, PDL2 | PD-1 | Co-inhibition | CD19+PD-L1+ B cells play a crucial role in driving SLE pathological responses and are directly correlated with various clinical indicators in SLE | CTLA-4 fusion protein and CTLA-4-Fc are under development |
| Sialic Acid | CD22(Siglec) | Co-inhibition | Loss of CD22 and Siglec-G leads to the development of anti-nuclear antibodies and lupus nephritis | Humanized CD22mAb has shown potential intervention effects in certain SLE patients |
Other B cell-related immune checkpoint molecules, including FCγRIIB and LAIR-1, are also believed to be associated with SLE.
Other key components in the immune system have also been implicated in the pathogenesis of SLE. Dendritic cells are responsible for mediating peripheral self-tolerance, while neutrophil extracellular traps are directly related to tissue damage in SLE patients.
Targeted drugs against these specific cells undoubtedly represent potential therapeutic strategies. In fact, drugs targeting PILRα have been shown to control tissue damage in SLE.
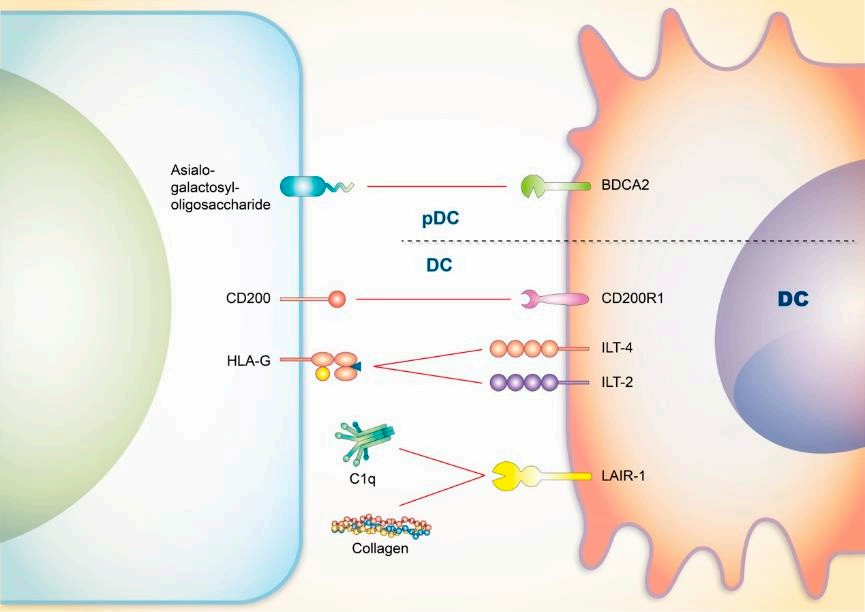 Fig.2. SLE and DCs.1,2
Fig.2. SLE and DCs.1,2
The enhancement of immune cells and co-stimulatory molecules is considered a crucial factor in the development of SLE. Conversely, it seems reasonable to intervene in SLE by manipulating dysregulated immune checkpoints, restoring specific pathways, and inhibiting pathogenic immune responses.
Many targeted drugs have demonstrated potential effectiveness against SLE, but their safety still needs further examination. Currently, successful clinical-stage targeted drugs primarily focus on the following immune checkpoint molecules:
Target
| CD80/CD86 | CD28 | ICOSL | VISTA | CD40 | CD40L | CD22 | BDCA2 |
Unfortunately, no drug has successfully reached the endpoint of Phase III clinical trials. However, immune checkpoint therapy for SLE has been proven to be an effective and feasible but still evolving approach. Considering the fundamental complexity of immune responses, multi-targeted drugs may represent a more comprehensive methodology.
At Creative Biolabs, we pride ourselves on providing comprehensive and unparalleled services to our valued customers. We understand that theoretical knowledge alone is not enough to meet the demands of our clients. That's why we go above and beyond by offering a wide range of services tailored to their unique needs and objectives.
Choose Creative Biolabs for a service experience that surpasses expectations. Our dedication, expertise, and comprehensive offerings set us apart from the competition. Let us take care of your needs and deliver the results you deserve.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.