Triple-negative breast cancer (TNBC) is a type of breast cancer that lacks the expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). Most of the TNBC are basal-like breast cancer, and the gene expression profiling and IHC data are different. The high expression of epidermal growth factor receptor (EGFR), CK5, caveolin-1, transmembrane glycoprotein NMB (GPNMB), and p63, have been confirmed in several TNBC subtypes. Moreover, recent reports have demonstrated that some proteins that mediate gene repair are expressed in TNBC, which might cause drug resistance in clinical applications. Also, a wide variety of signal pathways, such as mutagen activated protein (MAP) kinase pathway, poly ADP-ribose polymerase1 (PARP1) pathway, as well as Akt signal pathway, have been found to be associated with TNBC pathogenesis.
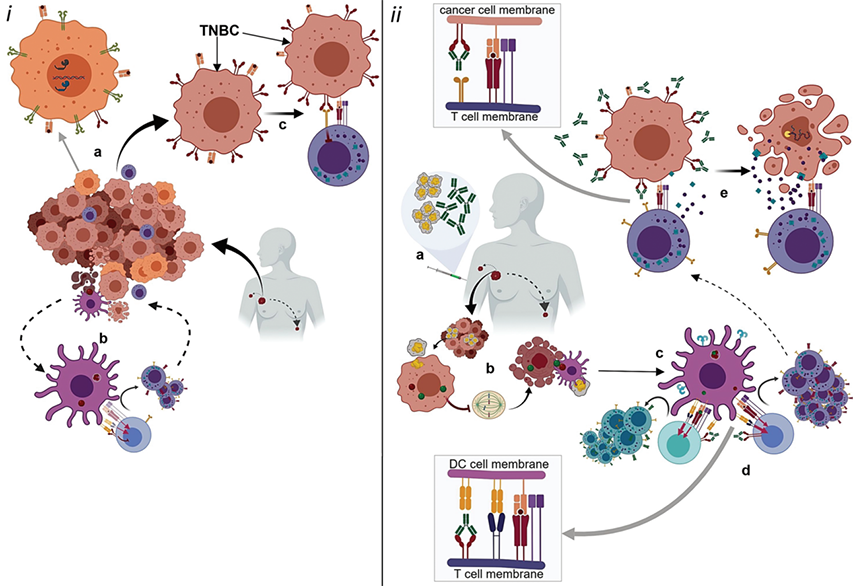 Fig.1. The possible mechanism of action of anti-PD-1/PD-L1 drugs.1,2
Fig.1. The possible mechanism of action of anti-PD-1/PD-L1 drugs.1,2
Recently, many studies have shown that patients with TNBC often have poor clinical outcomes and even relapse or die quickly. A battery of standard approaches, such as surgery, radiotherapy, as well as chemotherapy, in the treatment of TNBC have been generated and have become standard guidelines in clinical use. However, the results have suggested that these treatments are not very effective and do not prevent tumor recurrence.
As a result, a wide spectrum of targeted therapies, including antibodies and small molecule drugs, have been developed for the treatment of TNBC. For instance, several biomarkers, like EGFR and PARP, have been identified and evaluated for the treatment of TNBC both in pre-clinical and clinical trials. Additionally, a panel of new pathways, including NOTCH signaling pathways and Wnt/b-Catenin signaling pathways, plays a significant role in inhibiting TNBC progression and has also been regarded as promising therapeutic targets for TNBC treatment.
Nowadays, the treatment landscape for TNBC is changing with the discovery of immune checkpoints. PD-1/PD-L1 is a key immune checkpoint that is essential for various tumor pathogenesis. Moreover, current research has illustrated that PD-L1 expression is associated with the extent of tumor-infiltrating lymphocytes (TILs) infiltration in TNBC. Therefore, a range of immune checkpoint inhibitors (ICIs) target immune checkpoint protein PD-L1 has been designed for helping the immune system recognize and killing metastatic TNBC cells. The data have shown that the overall response rate can up to 20%, with median overall survival of 10-12 months.
Creative Biolabs is a global company that focuses on immune checkpoint discovery and drug development. With our extensive experience and unique platform, we can provide a series of immune checkpoint therapy services based on a wide variety of state-of-the-art technologies. Please do not hesitate to contact us for more information.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.