Kidney cancer caused more than 175,000 deaths in 2018, and its incidence is increasing worldwide. In the USA, kidney cancer is the eighth most common malignancy and accounts for 4.2% of new cancer diagnoses. Clear cell renal cell carcinoma (ccRCC) is the most common subtype, representing 60-80% of all primary kidney cancers. Advanced kidney cancer confers considerable morbidity and mortality, but advances in treatment have substantially improved the overall prognosis of patients with this disease in the past decade. In particular, immune checkpoint inhibitor (ICI) has proven to be an effective and important new strategy in the management of patients with kidney cancer.
Kidney cancer has several unique characteristics that make it an attractive disease for treatment with ICI. RCC is poorly responsive to conventional chemotherapeutics and, although treatments that target vascular endothelial growth factor (VEGF) have increased the number of therapeutic options, nearly all patients eventually acquire resistance to these molecularly targeted or antiangiogenic therapies. Besides, patients with metastatic RCC can show spontaneous regression of their disease without treatment, although this is rare. In addition, control of metastatic disease is sometimes seen after cytoreductive nephrectomy. These observations suggest strongly that a host-versus-tumor immune response might be present in some patients.
Increasing evidence now shows that RCC has a unique immune microenvironment compared with other solid tumors. An important area of the ongoing investigation is to adapt ICIs to these patient-specific and tumor-specific immune characteristics. With the advent of ICIs, we are entering a new era of systemic treatment of RCC. These new therapies have increased the potential of achieving lasting remissions and long-term survival in patients with metastatic disease, which was previously only possible for a small group of patients receiving high-dose IL-2. Single-agent inhibitors or combined inhibitors and combinations of ICIs with VEGFR TKIs are now established as part of the standard of care for advanced RCC. The choice between these ICIs-containing regimens remains unclear, pending further long-term data.
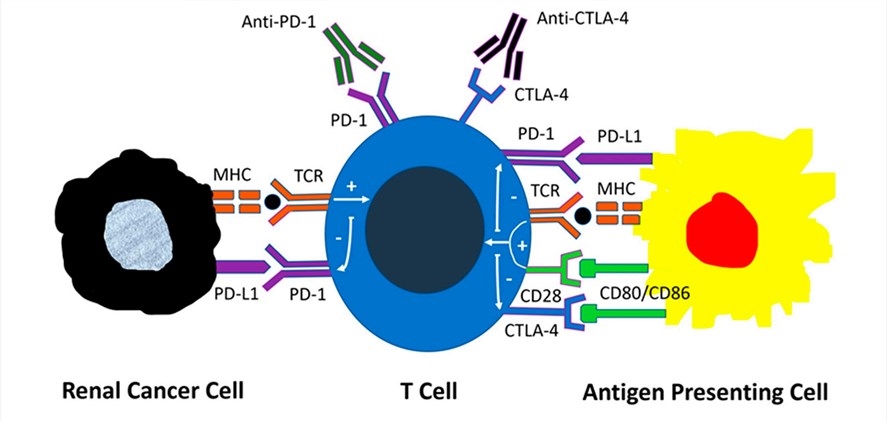 Fig.1. Mechanisms of action of immune checkpoint inhibitors.1,2
Fig.1. Mechanisms of action of immune checkpoint inhibitors.1,2
With an advanced technology platform and rich experience, Creative Biolabs is a professional immune checkpoint-based therapy development service provider. Having focused on this field for years, we are confident in providing a series of quality-assured customer services for the immune checkpoint-based kidney cancer therapy development, including but not limited to:
If you are interested in any of our services, please do not hesitate to contact us for more information.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.