Ovarian cancer is the most important cause of gynecological cancer-related mortality, with the majority of women presenting with advanced disease. The standard treatment for ovarian cancer includes surgery followed by platinum-based chemotherapy. Given the success of checkpoint inhibitors in the treatment of other cancers, there is an urgent need for new therapeutic strategies for ovarian cancer. Over the past decade, most of the focus of preclinical studies in ovarian cancer has been on the use of programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) as monotherapy. Currently, these immune checkpoint inhibitors have also been investigated in various combinations with chemotherapy, oncolytic vaccines, co-stimulatory molecules, poly ADP ribose polymerase (PARP) inhibitors, and other checkpoint inhibitors.
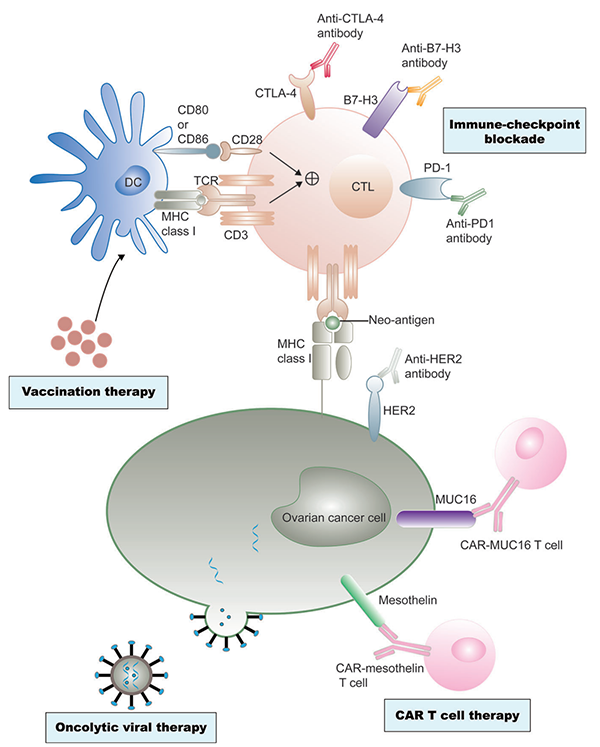 Fig.1. Current immunotherapy treatments of ovarian cancer.1,2
Fig.1. Current immunotherapy treatments of ovarian cancer.1,2
Adaptive immunity in ovarian cancer is rapidly expanding to enhance the dendritic cell-mediated presentation of ovarian cancer, predominantly by vaccination. Cytotoxic T lymphocytes (CTLs) are activated after recognizing tumor-associated antigens (TAAs), and particularly the neoantigens. Ovarian cancer tumor cells often evade destruction by CTLs through inhibitory signals in the tumor. In summary, the mechanisms of immunosuppressive networks of ovarian cancer include inhibition of CD8+ effector cells by Tregs, suppression of receptor PD-1 engaging by the ligand PD-L1, myeloid-derived suppressor cells, and inhibitory cytokines. Current immunotherapy treatments of ovarian cancer include immune checkpoint inhibitors, adoptive cell therapy (TCR-engineered T cells and chimeric antigen receptor T cells), cancer vaccines, and oncolytic viruses.
Based on the findings of preclinical studies, various agents blocking CTLA-4, PD-1, or PD-L1, or other immune molecules are currently investigated in ovarian cancer treatment. Details are shown in Table 1.
Almost all cancers, including ovarian cancer, have acquired similar capabilities during tumor progress via different mechanistic strategies. The hallmarks of cancers are an increasingly common problem faced by immunotherapies. Thus, the combination of immunotherapy with chemotherapy, radiation therapy, anti-angiogenesis drugs, and PARP inhibitors is essential.
Creative Biolabs is competent to present a flow chart aiming at developing efficient approaches to overcome the immunosuppressive tumor microenvironment to improve the efficacy of ovarian cancer immunotherapy. Creative Biolabs provides immune checkpoint antibody development, as well as immune checkpoint targeted small molecule drug development. If you are interested in our services, please feel free to contact us for more information.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.