Head and neck squamous cell cancer (HNSCC) is a type of squamous cell cancer found in the outer layer of skin and mucous membrane. HNSCC is classified by its occurrence site, such as the oral cavity, larynx, hypopharynx, as well as nasal cavity. Common symptoms of HNSCC include ulcers in the throat, abnormal bleeding in the mouth, unclear sinus congestion, sore throat, sore ears, pain and difficulty swallowing, hoarseness, or swollen lymph nodes. HNSCC can spread to other human body parts and has a worse prognosis. About 50% of patients with HNSCC survive only 3-5 years after diagnosis.
In recent years, pilot studies have indicated that HNSCC development is associated with serious immune defects. HNSCC cells can evade immune surveillance due to genetic variations and heterogeneity. Moreover, a wide variety of immune checkpoint molecules have been identified and used as therapeutic targets in the treatment of HNSCC. The data have suggested that these novel targets can trigger the activation of immune systems to provide durable responses.
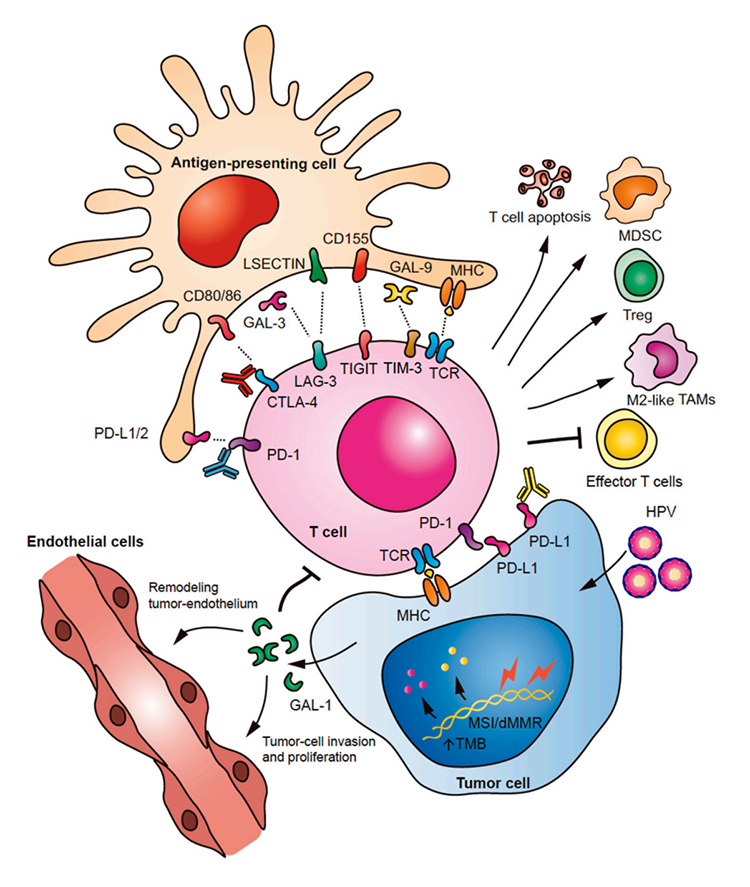 Fig.1. Immune checkpoints in HNSCC.1,2
Fig.1. Immune checkpoints in HNSCC.1,2
Over the last few years of studies, different molecular mechanisms and signaling pathways involved in HNSCC immune escape have been widely investigated. The results have illustrated that these pathways can induce T cell apoptosis, block T cell functions, and trigger the expansion of specific immunosuppressive tumor-infiltrating cells, like regulatory T cells (Tregs), tumor-associated macrophages (TAMs), or myeloid-derived suppressor cells (MDSCs). Besides, recent reports have revealed that the overexpression of galectin 1 (Gal-1) is associated with lower infiltration of T cells and has been considered a key prognostic factor in human tissue of HNSCC.
Understanding the important role of immune responses in cancer immunotherapy, many attempts have been made to develop new immune checkpoint therapies in the HNSCC treatment. Up to now, a battery of immune checkpoint molecules, including but not limited to CTLA-4, GITR, PD-1, and VISTA, have been regarded as effective therapeutic targets for generating novel HNSCC drugs.
For example, several PD-1 immune checkpoint inhibitors (ICIs) have been designed to hinder the inhibitory functions of PD-1 and its ligand, PD-L1. Numerous data have demonstrated that these inhibitors can improve patient outcomes in advanced or metastatic HNSCC. Furthermore, two PD-1 antibodies have also been examined and approved by FDA as a treatment for patients suffering from recurrent or metastatic HNSCC.
To develop a new class of cancer therapy, Creative Biolabs has established a well-mature immune checkpoint drug discovery platform to offers a series of immune checkpoint services, ranging from immune checkpoint molecule identification, immune checkpoint drug discovery to commercialization. Please feel free to contact us for more information.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2025 Creative Biolabs. All Rights Reserved.