Poliovirus receptor-like 2 (known as PVRL2, NECTIN-2, or CD 112), belongs to a nectin-like family involved in cell adhesion reactions and regulation of intracellular functions such as cell polarization, monocytic transmigration, homophilic hematopoietic, and endothelial adhesion through signal transduction. PVRL2 is expressed on the surface of hemopoietic (CD341, megakaryocytic, and myelomonocytic) cells and endothelial cells. Furthermore, a high level of expression of PVRIG was detected on CD45- cells, and CD14+ cells from multiple tumor types such as prostate cancer, ovarian cancer, renal carcinoma, lung cancer, endometrial carcinoma, and breast cancer. Normal lymphocytes do not express PVRL2. PVRL2 is a type-I membrane glycoprotein that belongs to the immunoglobulin superfamily (IgSF). Its ectodomain consists of a membrane-distal IgV-like domain and two IgC-like domains, followed by a single transmembrane region and a cytoplasmic tail.
PVRIG (PVR-related Ig domain, CD112R) is an emerging coinhibitory receptor of the PVRL2. In normal human blood, the PVRIG gene is expressed in T cells and NK cells, but not in neutrophils, DCs, B cells, and monocytes. The high-level expression of PVRIG was detected on NK cells, CD4+ and CD8+T cells from a wide battery of tumor types such as prostate cancer, ovarian cancer, renal carcinoma, lung cancer, endometrial carcinoma, and breast cancer. The PVRIG protein is about 36kD and includes one extracellular immune globulin variable-like (IgV) domain, a long endocellular domain, and a single transmembrane region.
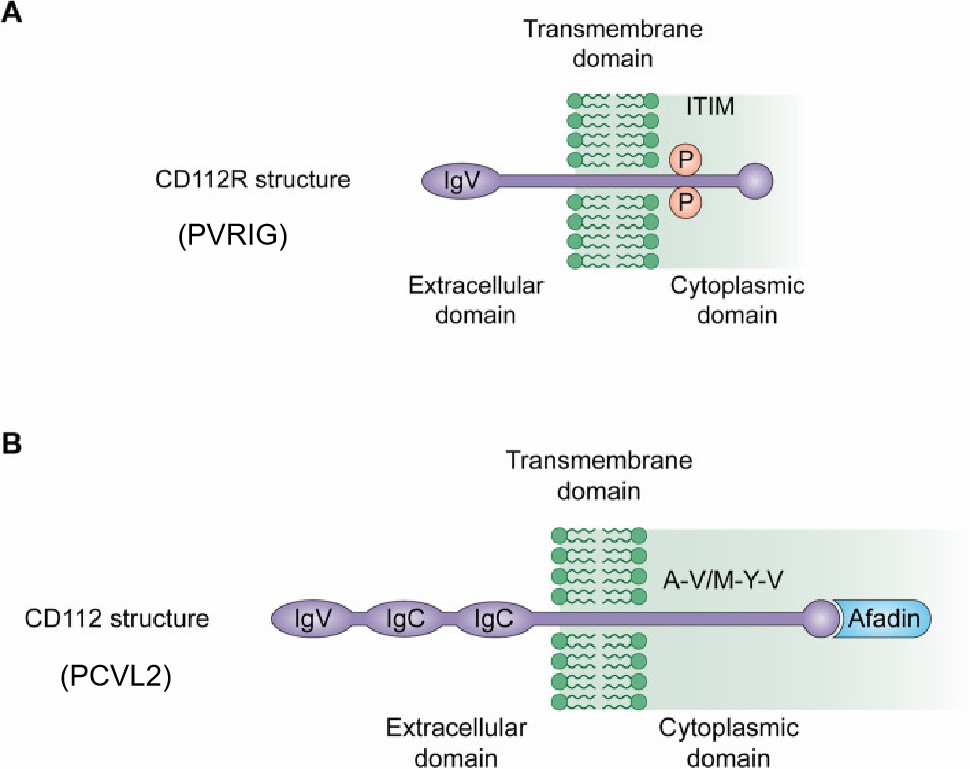 Fig.1 PVRIG and PCVL2 structures.1,2
Fig.1 PVRIG and PCVL2 structures.1,2
As the predominant inhibitory receptor of PVRL2, PVRIG's mRNA occurred on NK cells, CD4+, and CD8+ T cells in a battery of tumor types such as prostate cancer and breast cancer. Previous studies indicated that PVRL2 interacts with PVRIG to mediate immune-activating or inhibitory signaling in leukocytes, and T cell proliferation. While blockade of PVRIG can slightly increase cytokine production in CD4+ T cells and cell division, furthermore, enhances the CD8+ T cell-mediated cytotoxicity. For this reason, the PVRIG and PCVL2 pathway plays an important role in regulating the T cells kill tumor cells process. PVRL2 has been previously suggested to participate in the etiopathogenesis of acute myeloid leukemia via mediating extramedullary manifestations or influencing intracellular interactions between malignant and immunoreactive cells. Furthermore, an up-regulation of PVRL2 gene expression on hepatocellular carcinomas revealed that PVRL2 may participate in the inhibition of hepatoma cell apoptosis.
The potential of PVRIG and PCVL2 signaling as a tumor therapeutic target has been widely discussed in recent years. Presently, a highly reactive anti-PCVL2 drug which is in the initial clinical trial phase exerts its effects by preventing the co-inhibitory receptor PVRIG from binding to PVRL2. This trial is being conducted in breast cancer, ovarian cancer, and other solid tumors, and early signs of anti-tumor effectiveness have been reported.
Creative Biolabs is a worldwide company with extensive scientific expertise and has been a long-term expert in the field of immune checkpoints. If you are interested in immune checkpoints and looking for a reliable partner, Creative Biolabs will be your best choice. Our comprehensive services for worldwide customers include but are not limited to:
Please refer to our Services Overview for more information, and do not hesitate to contact us for more details.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.