Immune stimulating agents are substances that stimulate the immune system by activating or enhancing the activity of any of its components. In general, these immune stimulating agents work by activating and boosting the immune system to improve the recognition and killing of cancer cells when introduced into the tumor microenvironment or surrounding immune-rich tissues.
The combination of immune checkpoint therapy with immune stimulating agents is a promising approach in cancer treatment. Immune checkpoint therapies, such as immune checkpoint inhibitors (ICIs), aim to unleash the body's immune system to attack cancer cells by blocking inhibitory signals that dampen immune responses. However, not all patients respond to immune checkpoint therapy, and tumors can develop resistance mechanisms. To enhance the effectiveness of immune checkpoint therapy, researchers are exploring the use of immune stimulating agents in combination with ICIs.
The rationale behind combining immune checkpoint therapy with immune stimulating agents is to overcome the immunosuppressive tumor microenvironment and enhance the anti-tumor immune response. By simultaneously releasing the brakes on the immune system (through immune checkpoint inhibitors) and boosting its activity (through immune stimulating agents), this combination approach aims to improve treatment outcomes and increase the number of patients who benefit from immunotherapy.
Cytokines such as interleukin-2 (IL-2) and interferon-alpha (IFN-alpha) have been used to stimulate the immune response. IL-2 promotes the expansion of T cells and natural killer (NK) cells, while IFN-alpha enhances antigen presentation and T cell activation.
TLR agonists activate innate immune cells and promote the release of pro-inflammatory cytokines. These agents can help increase the recruitment and activation of immune cells in the tumor microenvironment.
Adjuvants can stimulate the immune system by enhancing antigen presentation and promoting the generation of anti-tumor immune responses. Combining these approaches with immune checkpoint therapy aims to improve the recognition of tumor-specific antigens by T cells and enhance the anti-cancer immune response.
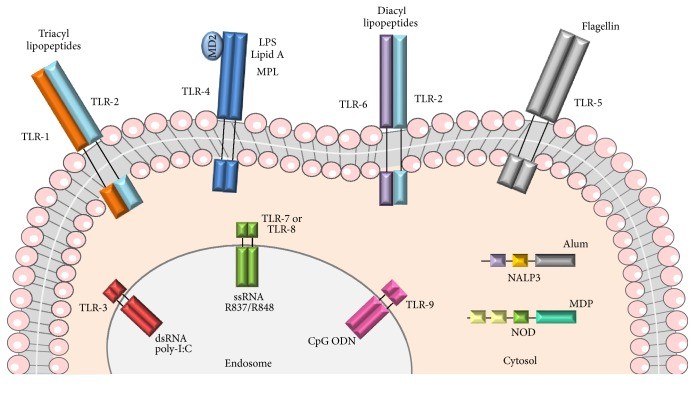 Figure 1 Adjuvants activate various immunological innate receptors.1,2
Figure 1 Adjuvants activate various immunological innate receptors.1,2
Oncolytic viruses are designed to selectively infect and kill cancer cells, leading to the release of tumor antigens and the activation of the immune response. They can enhance the efficacy of immune checkpoint therapy by promoting immune cell infiltration and antigen presentation.
Antagonistic mAbs can be utilized to downregulate or disrupt certain immune system processes that support tumor growth. In clinical settings, checkpoint blockade therapies based on this technique have gained great success. When PD-1 binds to its ligand, PD-L1, it inhibits T cell activation and proliferation, resulting in cell-cycle arrest. The use of anti-PD-1 and anti-PD-L1 mAbs to restore CTL-mediated anticancer effects has been intensively investigated in the clinic.
Creative Biolabs, a leader in immune checkpoint research and product development, offers a wide range of customized, high-quality services about the combination of immune checkpoint therapy with immune stimulating agents.
Please contact us for further information if you are interested in any of our services.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2025 Creative Biolabs. All Rights Reserved.