Anti-angiogenesis is another promising treatment strategy that tries to disrupt vascular supply and deprive the tumor of nutrition and oxygen, mostly by blocking the vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) signaling pathway. Vascular normalization can convert an immunosuppressive TME into an immune-stimulatory one by increasing immune effector cell accumulation, penetration, and anticancer activity while lowering hypoxia and suppressive cell function.
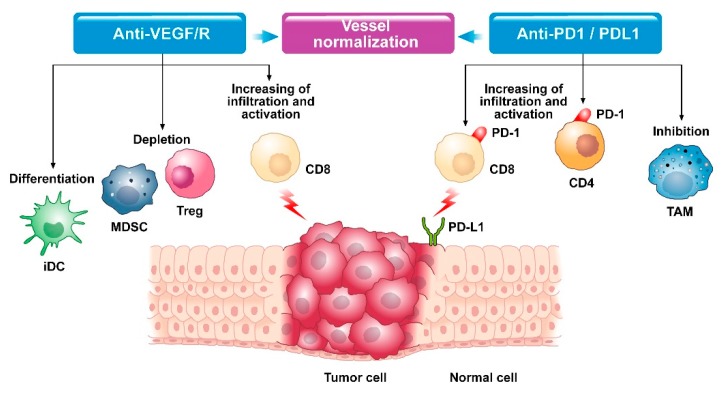 Fig.1. Modifications in the tumor microenvironment with anti-VEGF and anti-PD1/PDL1 therapy.1,3
Fig.1. Modifications in the tumor microenvironment with anti-VEGF and anti-PD1/PDL1 therapy.1,3
Anti-angiogenic drugs can improve antigen presentation and activation of cytotoxic CD8+ T cells, enhance lymphocyte infiltration and migration, reduce immunosuppression, and restore the host's robust anti-tumor immune response by interfering with the various stages of the cancer immunity cycle. Furthermore, restoration of the tumor vasculature improves antitumor immunity by increasing antigen presentation in DCs.
As immune checkpoint inhibitors (ICIs) promote breakthroughs in cancer immunity, the majority of patients develop ICI resistance. During anti-angiogenic therapy, intra-tumor hypoxia and associated immunosuppressive cell infiltration can also lead to angiogenesis relapse and drug resistance, and combining anti-angiogenic agents with chemotherapy or targeted therapies indicated little clinical advantages for the majority of cancer patients. Recent research suggests that combining ICIs with anti-angiogenic drugs could be a promising therapeutic method for improving the efficacy of ICIs.
Immunoenhancement and normalization of tumor vasculature are in a mutually reinforcing positive feedback loop. Immunotherapy restores the immune-supportive environment and promotes vascular normalization, enhancing lymphocyte activation and infiltration, whereas anti-angiogenic drugs block negative immune signals by raising the ratio of anti-tumor immune cells and decreasing the expression of immunological checkpoints. Combination therapy also helps to remodel the immunosuppressive environment, which improves the anti-tumor effects of both drugs.
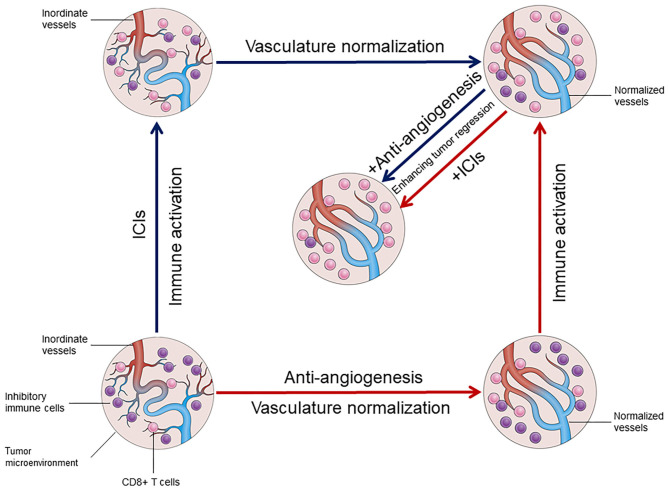 Fig.2. The interaction between vascular normalization and immune activation in the tumor microenvironment.2,3
Fig.2. The interaction between vascular normalization and immune activation in the tumor microenvironment.2,3
Several studies have demonstrated that VEGF-A or VEGFR inhibitors can have a synergistic anticancer impact when combined with anti-PD1 treatment. Most ICI with VEGFR tyrosine kinase inhibitors (TKIs) or anti-VEGF monoclonal antibodies combinations have shown a total survival improvement over single-agent antiangiogenic treatment. In advanced and pretreated RCC, the combination of an anti-VEGF monoclonal antibody to other PD-1/PD-L1 inhibitors demonstrated strong therapeutic efficacy. And, anti-VEGFR2 and anti-PD-1 combined treatment inhibited tumor growth more effectively than either treatment alone.
Creative Biolabs, with years of experience in immune checkpoint research and product development, offers a wide range of customized, high-quality services about the combination of immune checkpoint therapy with anti-angiogenic agents.
Please contact us for further information if you are interested in the services we provide.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.