A series of aberrant oncogenic signaling pathways, including mitogen-activated protein kinases (MAPK), have been indicated a role in promoting tumor progression. Targeting MAPK signaling pathways (e.g., BRAF, NRAS, and MEK) is a promising strategy for tumor treatment. Targeted therapies such as BRAF inhibitors or MEK inhibitors trigger remarkable and rapid clinical responses in most cancer patients. However, their duration of effect is relatively short due to the emergence of resistance.
Immune checkpoint therapy refers to immune checkpoint blockade, including anti-CTLA-4 blockade and anti-PD-1/PD-L1 blockade, a potential therapeutic strategy for multiple tumors. However, ICIs show long-term tumor regression, but the overall response rate was limited.
Combining BRAF inhibitors or MEK inhibitors with ICIs has been indicated to increase anti-tumor activity compared to monotherapy, with increased duration of response, prolonged survival rate, and overcoming resistance. The combination of BRAF inhibitors and MEK inhibitors increases the expression of melanoma antigens and HLA class I molecules in BRAF-mutant melanoma cell lines, which promote the uptake and processing of apoptotic melanoma cells by antigen presenting cells.
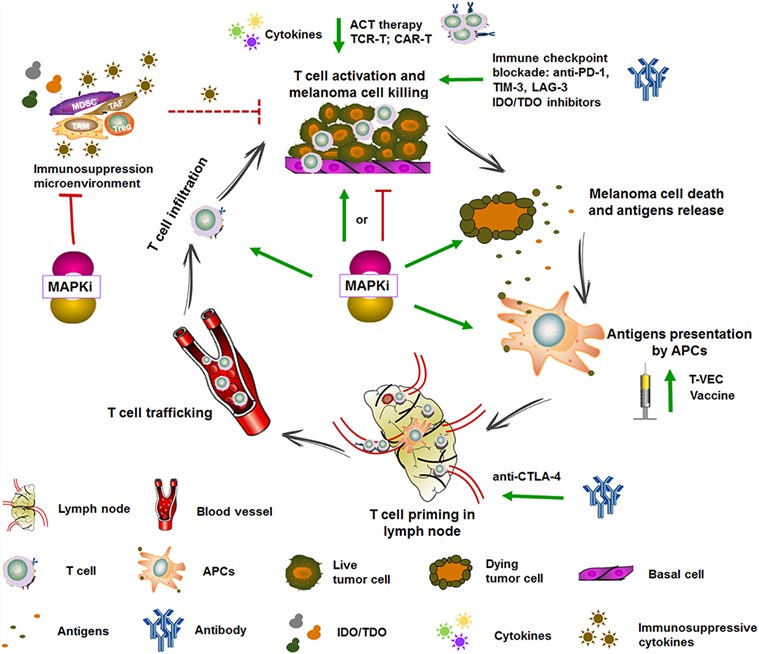 Fig.1. Targeted therapy and immunotherapy in the cancer-immunity cycle.1,2
Fig.1. Targeted therapy and immunotherapy in the cancer-immunity cycle.1,2
The combination of BRAF inhibitor and CTLA-4 blockade has been tested and evaluated in multiple preclinical studies. In mice models, the addition of BRAF inhibitor could significantly promote the expansion of antigen-specific CD8+T cells, enhancing the antitumor activity of CTLA-4 blockade. The combination of BRAF inhibition and PD-1 pathway blockade also exhibited a potent antitumor response through increasing the number and function of tumor-infiltrating T cells.
Based on these data, multiple clinical trials have been conducted to evaluate the safety and efficacy of the combination of targeted BRAF/MEK therapy and ICIs. Though some trials have shown encouraging results, there is no prospective data on appropriate treatments. Some clinical data suggest the combination of BRAF/MEK inhibitors and ICIs may trigger excessive severe toxicity.
The efficacy of ICIs is associated with the molecular smoking signature, higher neoantigen burden, and DNA repair pathway mutations. In vitro studies have reported that BRAF/MEK inhibitors in combination with checkpoint inhibitors, show pleasant outcomes. Currently, several clinical trials are ongoing to assess the efficacy, safety, and best dose of the combination of BRAF/MEK inhibitors and ICIs in NSCLC.
Clinical trials have been reported that MAPK inhibitors show a favorable clinical response in patients with thyroid cancer; however, the overall survival (OS) rate remains controversial, possibly due to the toxicity of the MAPK inhibitor and the resistance of the tumor microenvironment. Excitedly, some studies reported that the combination of MAPK inhibitors and ICIs may show favorable responses in thyroid cancer patients based on the immunomodulatory function of MAPK inhibitors.
A combination of ICIs and BRAF/MEK inhibitors has shown better therapeutic effectiveness and reduces toxicity in metastatic melanoma. Creative Biolabs has accumulated extensive experience in the field of ICI development. We are confident in providing a full range of immune checkpoint drug development services, including but not limited to:
If you are interested in our services, please contact us to discuss your project.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.