Mycobacterium tuberculosis is a leading cause of tuberculosis (TB), one of the top ten fatal diseases worldwide. Approximately 10.4 million people suffer symptomatic M. tuberculosis infections, and about 1.8 million people die of M. tuberculosis infections. M. tuberculosis infections mainly affect the lungs and can affect any other organ of the body. Treatment of TB depends on the type of infection and the drug sensitivity of Mycobacterium. People infected with drug-susceptible Mycobacterium can be cured with a standard 6-month course of four antimicrobial drugs. However, the prevalence of multi-drug-resistant tuberculosis (MDR-TB) requires developing a more effective therapy.
Immune checkpoints are a series of co-stimulatory and inhibitory signals for regulating antigen recognition. It plays an essential role in the immune system, regulating cell survival, cell cycle progression, and differentiation to effector and memory cells. Immune checkpoints include a variety of immune checkpoint proteins such as programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1), cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), and mucin domain-containing protein 3 (TIM-3). These proteins, acting as costimulatory or inhibitory proteins, are involved in the progression of multiple infectious diseases.
For TB, immune checkpoint proteins have been revealed a role in pathological progress. CD4+ T cells may be involved in host resistance to M. tuberculosis. TB-specific CD4+ T cells in individuals with active TB infection have been shown to secrete IFNγ, IL-2, and TNF and express PD-1. The levels of PD-1 on total CD4+ T cells of actively infected individuals were slightly higher than those of healthy donors. While some in vivo studies used mice as models showed PD-1 deficiencies might contribute to TB pathology. The lungs of the PD-1-deficient mice revealed uncontrolled bacterial proliferation and areas of focal necrosis, with predominantly neutrophilic infiltrates but with a small number of T and B cells.
The treatment of MDR-TB is complicated. Patients with MDR-TB have to be treated with the second-line and third-line drugs for long periods. The emergence of immune checkpoint therapy has shaped the therapeutic landscape of some MDR-TB. Combining these drugs with checkpoint inhibition may allow immunity to develop when the bacterial burden is under even partial control. In mice with Mycobacterium TB, inhibition of PD-1 and PD-L1 could increase levels of AKT and mTOR, along with decreased levels of TNF-α, NF-κB, IL-17, IL-2, IL-6, IL-17A, and IFN-γ, enhancing the innate immune response of alveolar macrophages to Mycobacterium TB.
Nevertheless, immune checkpoint inhibitors for treating Mycobacterium TB have yielded conflicting results. A recent study showed that lung cancer patients treated with immune checkpoint inhibitors could relieve the symptoms of lung cancer but increase the incidence of active TB. However, the study also showed that cancer patients treated with immune checkpoint inhibitors could not increase the risk of TB in a non-endemic TB area.
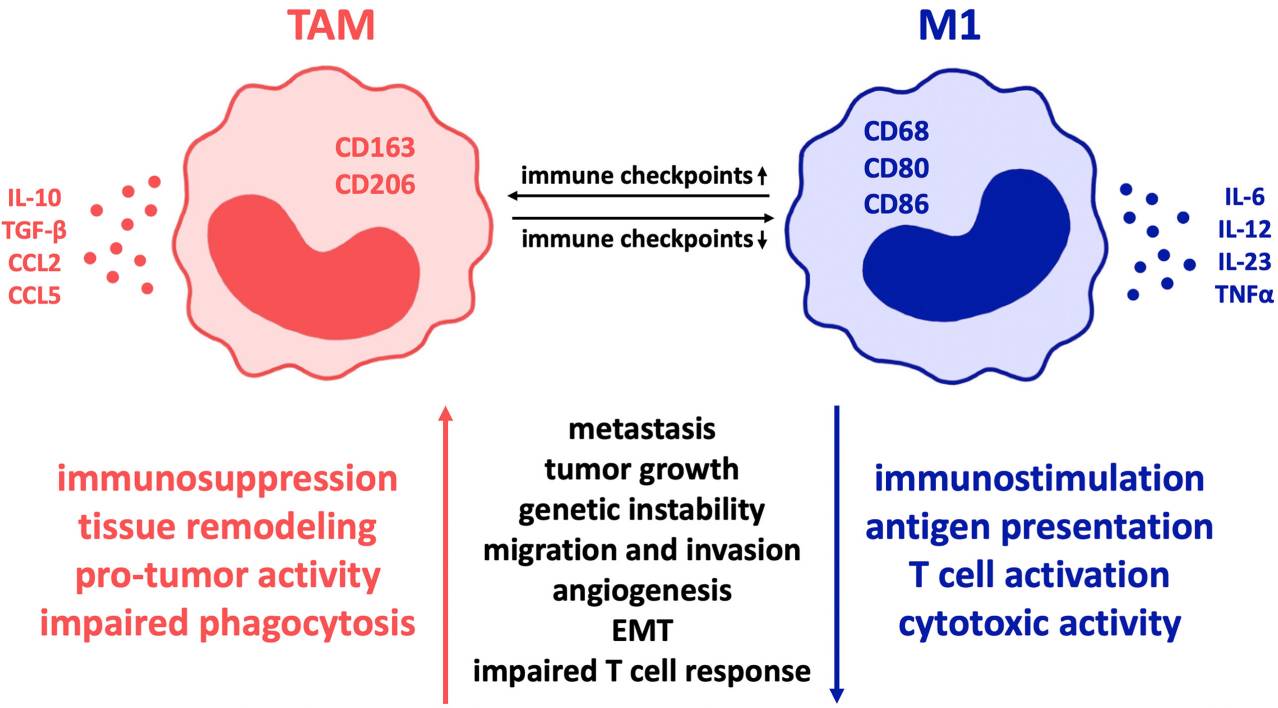 Fig.1. Influence of co-regulatory immune checkpoint molecules on macrophage polarization in cancer.1,2
Fig.1. Influence of co-regulatory immune checkpoint molecules on macrophage polarization in cancer.1,2
Equipped with world-class technologies and comprehensive expertise, Creative Biolabs is specialized in providing a widely full range of immune checkpoint therapy services for global clients, facilitating their infectious diseases therapy research, including Mycobacterium TB. Our service scale-up packages include custom immune checkpoint inhibitor development such as antibodies, proteins, peptides, small molecule drugs, and in vivo and in vitro drug assay services. If you are interested in our services, please contact us to discuss your project.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.