Adaptive immune resistance denotes a phenomenon whereby cancer undergoes a phenotypic transformation in reaction to a cytotoxic or pro-inflammatory immune reaction, consequently enabling it to circumvent said response. This adaptive mechanism is set in motion by the specific identification of cancerous cells by T cells, ultimately leading to the generation of cytokines that stimulate the immune system. Subsequently, cancer cells exploit defensive mechanisms designed to constrain inflammatory and immune reactions, effectively shielding themselves against T cell assaults. Creative Biolabs provides research about adaptive immune resistance, which may hold significance in the development of alternative strategies for cancer immunotherapy.
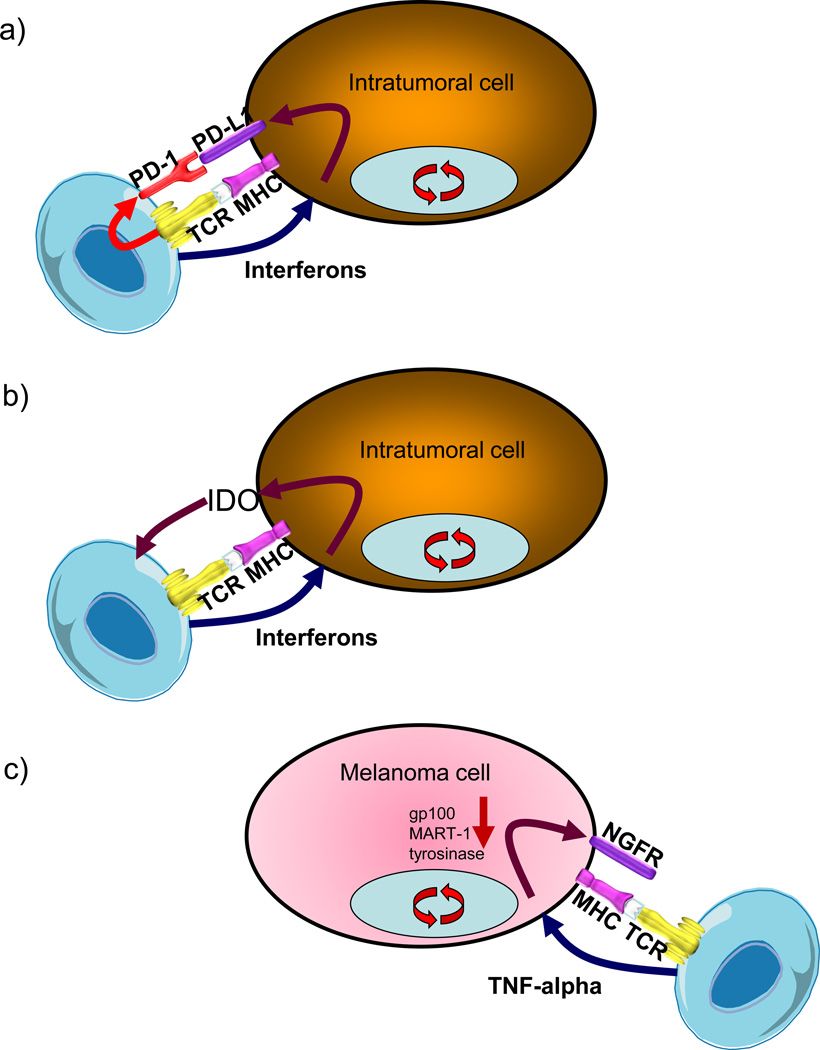 Fig. 1 Exemplars of adaptive immune resistance.1
Fig. 1 Exemplars of adaptive immune resistance.1
Prostate cancer is classified as a "cold" tumor primarily due to its relatively low mutation burden in comparison to "hot" tumors like melanoma and non-small cell lung cancer (NSCLC). The limited mutational load in prostate cancer results in a scarcity of antigens available for recognition by T-cells, leading to a paucity of tumor-infiltrating T-cells. Consequently, prostate cancer has exhibited notable resistance to immune checkpoint therapy (ICT). The principal resistance mechanisms to ICT in prostate cancer involve the exclusion of T-cells from the tumor microenvironment (TME) or the failure to induce substantial T-cell infiltration. The absence of tumor-infiltrating T-cells poses a substantial barrier to achieving clinical benefits through the blockade of T cell-inhibitory pathways like PD-1/PD-L1.
Therefore, a more targeted and concise approach to the design of the phase III combination trial could have proven advantageous. Such an approach would have involved a smaller clinical trial focused on a comprehensive assessment of the androgen receptor antagonist's potential to enhance the infiltration of T-cells into the TME and subsequently elevate the expression levels of PD-1 and PD-L1. Similarly, a phase III combination study that integrated anti-CTLA4 therapy with radiotherapy for patients with metastatic prostate cancer also experienced a shortfall in achieving its primary endpoint. The root cause of this setback can be attributed to a limited understanding of the immunological consequences arising from the administration of anti-CTLA4 or radiotherapy within the prostate TME. This knowledge gap extends to the sites of metastatic disease, which predominantly manifest as bone metastases in patients afflicted with metastatic prostate cancer.
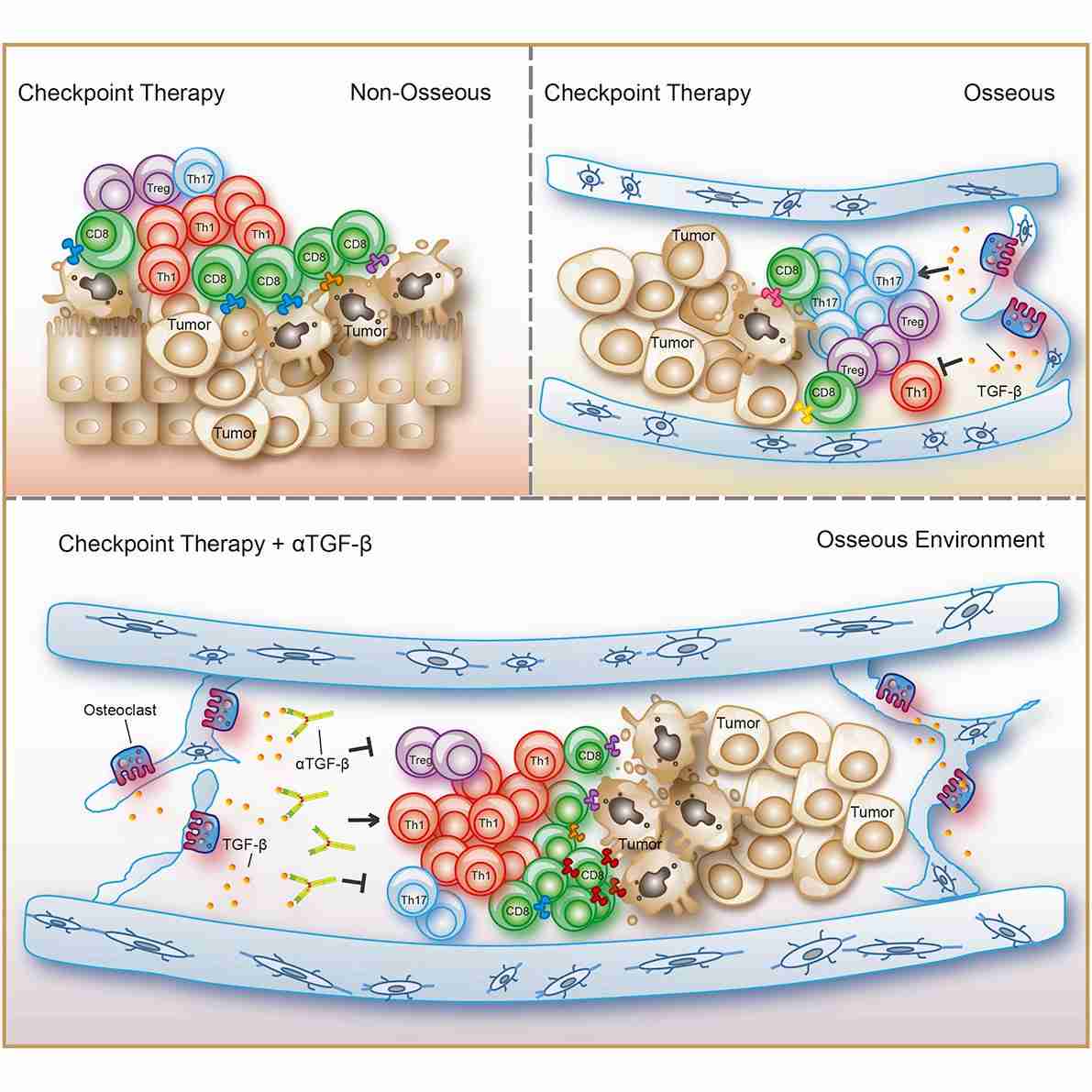 Fig. 2 Variations in the TME shape T helper lineage polarization and influence ICT response.2
Fig. 2 Variations in the TME shape T helper lineage polarization and influence ICT response.2
Anti-CTLA4 exhibited the capability to augment the infiltration of T cells within the tumor. Nevertheless, the administration of anti-CTLA4 concurrently prompted an escalation in compensatory inhibitory mechanisms, commonly referred to as adaptive resistance mechanisms. These mechanisms entailed an elevated presence of cells expressing PD-1/PD-L1 and VISTA within the TME. VISTA serves as an inhibitory checkpoint with pronounced expression on myeloid cells, including macrophages, dendritic cells (DCs), monocytes, and neutrophils, and moderate expression on T cells. This duality in immune responses, wherein therapy may stimulate antitumor immune reactions while simultaneously bolstering immunosuppressive mechanisms, constitutes a pivotal concept to consider during the development of novel immunotherapeutic strategies.
Creative Biolabs uses the invaluable insights derived from these preclinical data, it becomes imperative to confront the concept of organ-specific TME as the field of ICT advances over the ensuing decade. Consequently, tailoring distinct immunotherapeutic strategies tailored to these organ-specific contexts will emerge as an essential consideration in optimizing treatment outcomes.
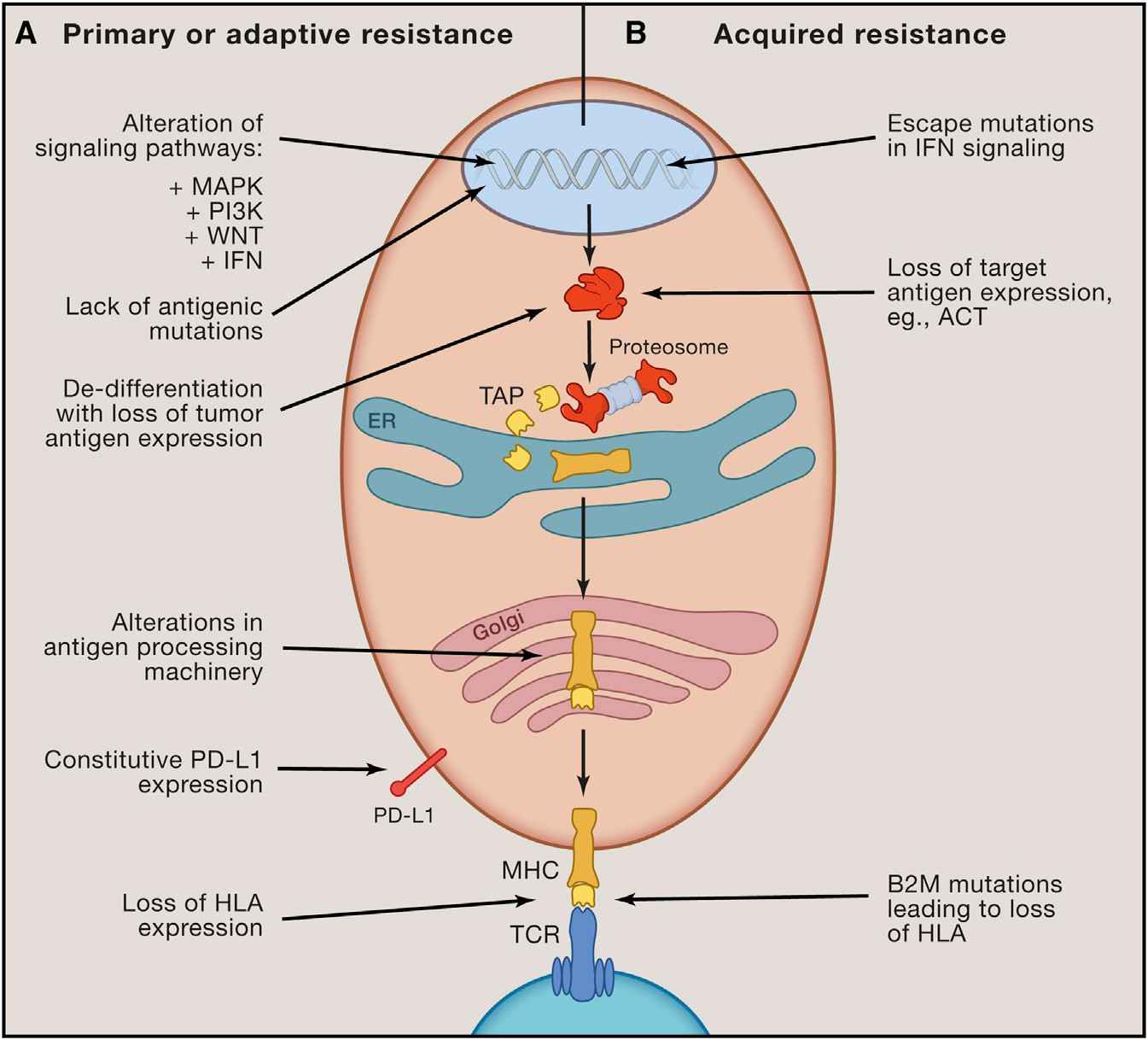 Fig. 3 Recognized inherent mechanisms of immunotherapy resistance.3 |
Immunotherapy for cancer has the potential to elicit enduring responses in individuals afflicted with metastatic cancers spanning various histological origins. Extending the clinical utility of these therapies necessitates an enhanced comprehension of the constraints that impede cancer immunotherapy. The interactions between the immune system and cancer cells are characterized by perpetual, dynamic, and evolving dynamics-from the inception of cancer cells to the progression into metastatic disease, where immune evasion becomes pivotal. As we gain insight into the molecular underpinnings of immunotherapy resistance, actionable approaches for mitigation or intervention may emerge, thereby enhancing the overall clinical prospects for patients. |
Creative Biolabs provides a comprehensive range of tailored services pertaining to immune checkpoints, encompassing, yet not confined to: Biomarker Development for Immune Checkpoint Inhibitor (ICI), Immune Checkpoint Assays, Preclinical Research for Immune Checkpoint Targeting Drugs, etc. Please contact us for a further information.
References
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2026 Creative Biolabs. All Rights Reserved.