A Novel Mechanism for Enhancing Checkpoint Blockade in Cancer Immunotherapy - sCTLA-4
CTLA-4 is an important immune checkpoint receptor that is involved in the maintenance of immune homeostasis, tolerance and tumor control in the body. CTLA-4 antibodies are expected to be used as therapies for the treatment of many human cancers. Recently, scientists from the University of Liverpool and other institutions have identified a potential new approach that may help and develop better cancer therapies - sCTLA-4, a selectively spliced soluble mutant encoded by the CTLA4 gene.
Creative Biolabs describes research on sCTLA-4, which explores its functional properties and potential impact effects on cancer immunotherapy. With our advanced platform, we are committed to being the best immune checkpoint research service provider and establishing the most efficient CTLA-4-based drug development for our global clients, including but not limited to:
sCTLA-4: A Neglected Form of CTLA4
CTLA-4 is a crucial negative regulator of T cell activation and proliferation, primarily expressed on the surface of activated T cells. It competes with the co-stimulatory molecule CD28 for binding to its ligands, CD80/CD86, on antigen-presenting cells (APCs), thereby inhibiting T cell activation and promoting immune tolerance. Dysregulation of CTLA-4 signaling has been implicated in various autoimmune diseases and cancer, highlighting its significance in immune homeostasis and anti-tumor immunity.
In the search for new strategies to enhance checkpoint blockade, researchers made a remarkable discovery - sCTLA-4. This form of CTLA-4 exists outside cells, freely circulating in bodily fluids and is thereby available for therapeutic actions much more directly and swiftly compared to its membrane-bound counterpart. Unlike membrane-bound CTLA-4, which acts as an inhibitory receptor for T-cells, sCTLA-4 acts as a decoy receptor, sequestering key immunosuppressive molecules and amplifying anti-tumor immune responses.
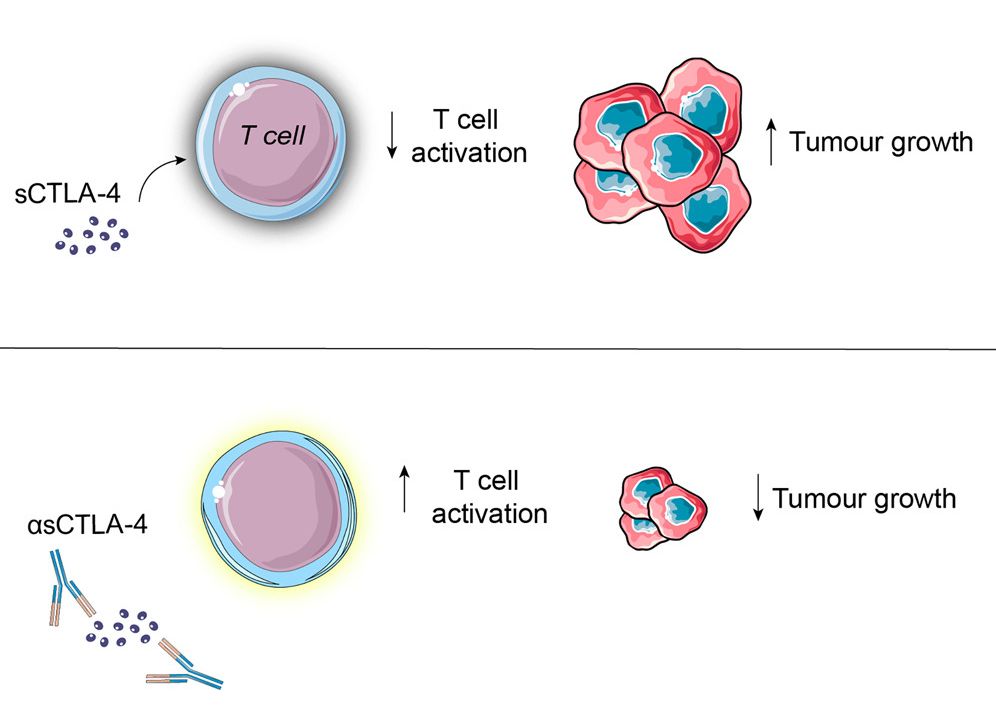 Fig. 1 Targeting sCTLA-4 promises to enhance checkpoint blockade cancer immunotherapy.1
Fig. 1 Targeting sCTLA-4 promises to enhance checkpoint blockade cancer immunotherapy.1
-
The mechanism of action of sCTLA-4 involves its ability to bind to ligands such as CD80 and CD86, which are also targets of membrane-bound CTLA-4. By competitively binding to these ligands, sCTLA-4 prevents the interaction between membrane-bound CTLA-4 and its ligands, thereby attenuating the inhibitory signals delivered to T cells.
-
In addition, sCTLA-4 has been shown to disrupt the formation of immunosuppressive regulatory T cells (Treg), further enhancing the anti-tumor immune response.
The soluble nature of sCTLA-4 offers significant advantages over traditional CTLA-4 blocking therapies.
-
Unlike monoclonal antibodies that have a limited half-life and require repeated administration, sCTLA-4 can be designed for extended circulation to provide sustained immune modulation with less frequent administration.
-
The decoy function of sCTLA-4 mitigates the systemic toxicity associated with conventional CTLA-4 blockade, potentially expanding its therapeutic window and improving patient tolerability.
Mechanisms of Action
sCTLA-4 acts through multiple mechanisms to enhance checkpoint blockade and anti-tumor immune responses.
-
Competitive inhibition: By binding to B7 ligand, sCTLA-4 competitively inhibits the interaction between B7 ligand and membrane-bound CTLA-4, thereby preventing down-regulation of T cell activation.
-
Amplification of costimulatory signals: In addition to blocking CTLA-4-mediated inhibitory signals, sCTLA-4 enhances CD28-mediated costimulatory signals and promotes robust activation and effector functions of T cells.
-
Immunomodulation: sCTLA-4 can regulate the tumor microenvironment by altering the balance of immune cell subpopulations, promoting the infiltration of effector T cells, while suppressing Treg and myeloid-derived suppressor cells (MDSC).
-
Systemic effects: Unlike anti-CTLA-4 antibodies that can cause systemic immune activation and toxicity, sCTLA-4 can exert more local effects in the tumor microenvironment, thereby minimizing off-target effects.
Preclinical and Clinical Insights on sCTLA-4
There is now much compelling preclinical and clinical evidence confirming the promise of sCTLA-4 as a novel mechanism to enhance checkpoint blockade.
|
|
Innovations
|
|
Preclinical Research on Immune Checkpoint Drugs
|
-
In preclinical models of multiple cancers, including
-
Melanoma
-
Lung cancer
-
Colorectal cancer
sCTLA-4 showed potent antitumor activity, leading to tumor regression and prolonged survival.
-
Administration of recombinant sCTLA-4 protein or gene therapy vectors encoding sCTLA-4 inhibits tumor growth, improves survival outcomes, and enhances the effectiveness of checkpoint blockade therapy.
-
The combination of sCTLA-4 with anti-CTLA-4 or anti-PD-1 mAb enhanced tumor regression and improved survival outcomes compared to monotherapy alone.
-
Combination treatment of sCTLA-4 with anti-PD-1 or anti-CTLA-4 antibodies synergistically enhances T cell activation, cytokine production and tumor infiltration, leading to tumor regression and long-term survival in a subset of treated animals.
|
|
Clinical Studies
|
Early-stage clinical trials are investigating the use of recombinant sCTLA-4 proteins or gene therapy vectors encoding sCTLA-4 in patients with advanced solid tumors, including melanoma, non-small cell lung cancer, and renal cell carcinoma.
-
In a Phase I trial evaluating the safety and efficacy of sCTLA-4 in patients with advanced solid tumors, preliminary data demonstrated good tolerability and promising anti-tumor activity.
-
The safety profile of sCTLA-4 appears to be favorable, with a lower incidence of immune-related adverse events compared to conventional CTLA-4 inhibitors.
|
Challenges and Future Directions
While the potential of sCTLA-4 in cancer immunotherapy is promising, a number of challenges and questions remain to be addressed.
-
One challenge is to elucidate the precise mechanisms of action of sCTLA-4 immunomodulatory effects and its interactions with other immune receptors and signaling pathways. A deeper understanding of these mechanisms could inform the development of more targeted and effective therapeutic strategies.
-
Another challenge is to optimize the delivery and dosage of sCTLA-4 to maximize its therapeutic efficacy while minimizing potential side effects. Strategies to enhance the stability, specificity, and tumor-targeting ability of sCTLA-4 formulations are being actively explored to improve their clinical utility and safety.
-
In addition, the heterogeneity of the tumor immune microenvironment and the complex interactions between immune cells and tumor cells pose additional challenges for the successful implementation of sCTLA-4 therapies in clinical practice. Personalized approaches that take into account individual differences in tumor biology, immune profiles, and treatment response are essential to achieve optimal outcomes with sCTLA-4-based immunotherapies.
In future studies, the development of novel delivery systems and formulations to enhance the pharmacokinetic properties of sCTLA-4 and to improve its bioavailability and tissue penetration in the tumor microenvironment could be considered. In addition, combinatorial approaches combining sCTLA-4 with other immunomodulators, such as cytokines or lysoviruses, are also expected to further enhance anti-tumor immune responses.
Through its unique mechanism of action and enhanced specificity, sCTLA-4 holds promise in overcoming the limitations of traditional CTLA-4 inhibitors and maximizing the therapeutic potential of checkpoint blockade therapy. Creative Biolabs, with its commitment to innovation and excellence, stands at the forefront of immune checkpoint services. You can contact us with any research ideas or needs you may have.
Reference
-
Kennedy, Paul T., et al. "Soluble CTLA-4 attenuates T cell activation and modulates anti-tumor immunity." Molecular Therapy (2024).
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
 Fig. 1 Targeting sCTLA-4 promises to enhance checkpoint blockade cancer immunotherapy.1
Fig. 1 Targeting sCTLA-4 promises to enhance checkpoint blockade cancer immunotherapy.1