Butyrophilins (BTNs) are a group of immunoglobulin glycoproteins that play an important role in mediating immune responses against different pathogens. In humans, the BTN family members can be classified into three subtypes, BTN proteins, BTN-like proteins, as well as SKINT-like proteins. BTN proteins contain BTN1A1, BTN2A1, BTN2A2, BTN2A3, BTN3A1, BTN3A2 and BTN3A3. BTN-like proteins are composed of BTNL2, BTNL3, BTNL8, BTNL9, and BTNL10. They can be expressed on a battery of cell types, such as T cells, dendritic cells (DCs), as well as neutrophils. Meanwhile, BTN can be easily found on the milk fat globule membrane (MFGM) protein and is critical for regulating milk fat secretion in bovine.
Besides, recent reports have demonstrated that most BTN and BTN like molecules can inhibit the proliferation and activation of T cells by interrupting cell cycle processes. For instance, BTN proteins and their antibodies can modulate a series of T cell functions, like the increment or reduction of T cell activation, differentiation, and T cell localization. Moreover, many researchers have revealed that BTNs are associated with a wide variety of autoimmune diseases and inflammatory diseases. Therefore, they can be treated as therapeutic targets and have broader clinical applications.
| Checkpoint receptor | Member | Cell type affected | The function of ligand-receptor interaction |
| Butyrophilins | BTN1A1, BTN2A1, BTN2A2, BTN2A3, BTN3A1, BTN3A2, BTN3A3, BTNL2, BTNL3, BTNL8, BTNL9, BTNL10, SKINT | Lymphocytes, dendritic cells, monocytes, macrophages, neutrophils, eosinophils | Co-stimulatory or co-inhibitory |
BTN is a type I transmembrane glycoprotein of about 25.4 kDa and consists of several disulfide bonds, two extracellular immunoglobulin domains. The extracellular immunoglobulin domains include an IgV-like and an IgC-like domain. Furthermore, recent studies have reported that several newly discovered BTN molecules have an intracellular B30.2/SPRY domain, which is essential for the production of mile fat. A conserved domain encoded by B30.2 (PRYSPRY) has also been identified in most BTNs, which is involved with alternative splicing. In particular, BTN1A, BTN2A, and BTN3A have the same structures. BTNL3, BTNL8, and BTNL9 share the similar structure of two extracellular immunoglobulin domains and a B30.2 domain.
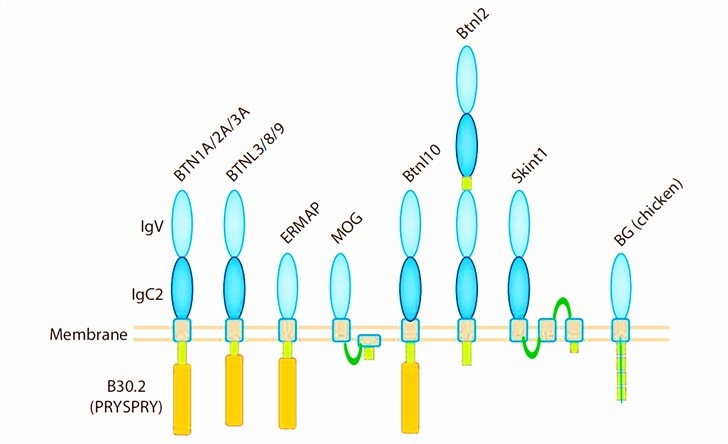 Fig.1 Protein domain structure of butyrophilins. (Rhodes, 2016)
Fig.1 Protein domain structure of butyrophilins. (Rhodes, 2016)
In the past few years, a subset of BTN family molecules, such as BTN1A and BTN2A, have been discovered and broadly studied in preclinical and clinical trials. Many research and drug development efforts targeting BTN family members have been made to the treatment of autoimmune diseases and cancers. These drugs are designed based on the co-stimulatory/co-inhibitory immunomodulatory mechanism of BTN members. For example, numerous studies have been carried out to improve co-stimulatory or reduce the co-inhibitory effect to trigger strong immune responses against various tumor types, such as lung cancers and breast cancers. Nowadays, a wide variety of novel drugs that target BTN members, like antibodies, small-molecule drugs, have been generated and evaluated in many disease models.
As a professional expert in the field of immune checkpoint-based drug discovery, Creative Biolabs offers a comprehensive collection of butyrophilin-related services for our clients to regulate immune responses, determine potential binding partners for different butyrophilin family members, as well as discover novel drugs for immune checkpoint blockade.
Reference
All listed customized services & products are for research use only, not intended for pharmaceutical, diagnostic, therapeutic, or any in vivo human use.
USA
Tel:
Fax:
Email:
Copyright © 2024 Creative Biolabs. All Rights Reserved.